The experience with Chinese COVID-19 Vaccine
The experience with Chinese COVID-19 Vaccine (New Coronavirus Vaccine)
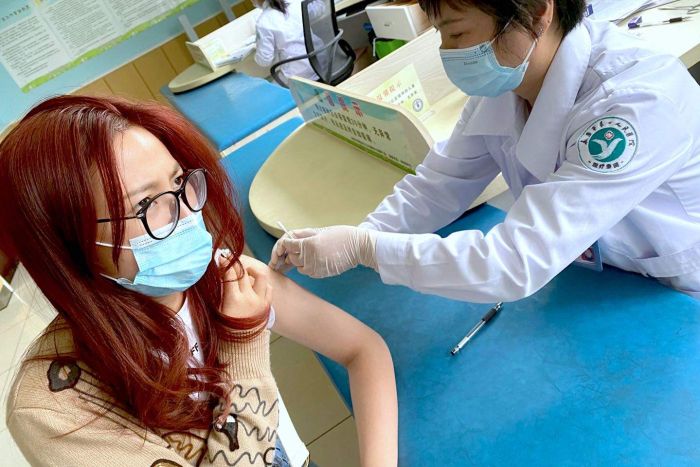
A British international university student from China: Wu Yilu shared her experience with Chinese COVID-19 vaccine (New Coronavirus Vaccine). In mid-October, Wu Yilu woke up and turned on her mobile phone and screamed happily. She received a text message notification to become the first batch of vaccinators for the third phase of China’s COVID-19 vaccine trial.
Wu Yilu, 20 years old this year, is studying finance in the UK. She said that she did not hesitate to join the third phase of the trial, hoping to return to the UK to return to the right track of life after the vaccine.
Two days later, Wu Yilu received the first shot of the candidate vaccine developed by the Chinese company Sinovac at a health center in Jinhua, Zhejiang. The second shot will be given next week.
In the global COVID-19 vaccine competition, China has four candidate vaccines that have entered phase III clinical trials among the forefront, but they have not yet received final marketing approval.
According to a report issued by the Chinese government in October, more than 60,000 Chinese citizens who have been vaccinated “have not reported serious adverse reactions.”
Following Wu Yilu’s vaccination experience, the vaccination details of several vaccines began to enter the public’s field of vision.
How effective is China’s vaccine trials?
In mid-October, Wu Yilu submitted her vaccination registration information through the WeChat applet, and provided her own air ticket and UK visa to prove that her conditions meet the requirements of the vaccination population: international students who plan to go abroad.
At first, she had concerns about the safety and quality of the vaccine, but she later believed that the number of new cases reported in the UK recently exceeded 20,000. If the vaccine is not vaccinated, returning to the UK may be even more dangerous in the future.
“I am in a very emotional mood. It feels like a sunny day after a rainy day in the UK, and I feel hopeful,” she said.
Wu Yilu spent nearly 100 Australian dollars (about 550 yuan) to purchase two COVID-19 vaccines. Before the formal vaccination, she needs to sign a consent form and communicate with the doctor her history of disease and allergy.
In this copy of the consent form obtained by ABC, the details of the vaccination plan for the COVID-19 vaccine developed by the Chinese pharmaceutical company Kexing are disclosed to the public for the first time.
According to this plan, local Chinese citizens aged 18 to 59 are eligible to receive two vaccine injections within 14 to 28 days. People who are allergic to candidate vaccine components and those who have been infected with the COVID-19 virus are advised not to participate in the trial.
Participants were told that common side effects such as skin irritation or temporary fever would occur within 24 hours of receiving the trial vaccine.
At the same time, the document also pointed out that vaccinators may have rare adverse reactions, including skin rash and severe fever.
Wu Yilu said that her first injection was “painless” and within 30 minutes after the vaccination, she successfully completed the observation process and was told by the doctor that she could leave.
“I feel that I have an extra layer of protection than others,” she said. “I didn’t have any bad reactions, vomiting, dizziness and wanting to sleep, and I didn’t even have a fever. I just lived a normal life.”
In 2018, the vaccine safety scandal that broke out in China caused many Chinese people to feel disappointed in domestic vaccines. After the outbreak of the COVID-19 epidemic, most people took a wait-and-see attitude towards these vaccines that have not yet passed the final test.
But Wu Yilu said that she wanted to be “the first person to eat crabs” because she wanted to use her own experience to encourage others.
So, after she was vaccinated, she posted her experience and experience on social media Xiaohongshu.
“After so long of hard work, China has spent so much effort to develop such a small vaccine. As a Chinese, I am very proud of it,” she said.
d thousands of comments, private messages and likes, and there were voices of support and criticism.
Critics reminded her that she was “as an experimental mouse”, but there were also a large number of international students who supported her courage and asked her for details.
Since the outbreak of the COVID-19 epidemic in Wuhan, the epicenter of the epidemic in China, at the beginning of this year, there have been more than 48 million confirmed cases worldwide and the death toll has exceeded 1.2 million. This makes the world’s expectation for effective vaccines even more urgent.
Like many countries’ vaccination programs, China prioritizes high-risk groups, including medical staff, customs officials, and overseas travelers. International students are also one of these groups.
Zheng Zhongwei, head of the China New Coronavirus Vaccine Research and Development Working Group, said that it is expected that by the end of this year, the output of China’s domestically produced new coronavirus vaccine will reach 610 million.
Currently, there are 13 vaccines in clinical trials in China.
Four candidate vaccines from Sinopharm and Sinovac have entered phase III trials, with participants in 10 countries including Brazil, Indonesia, Turkey and Russia.
According to Xinhua News Agency, China’s official news agency, Indonesia announced plans to vaccinate 9 million people with Kexing Biotech’s candidate vaccine starting in mid-December, thereby promoting Bali’s development as a “green zone” early next year.
But at the same time, this vaccine was protested in Brazil, with more than 300 people protesting on the streets of Sao Paulo, Brazil against the vaccine trials conducted there, and the governor of Sao Paulo’s support for mandatory vaccination of this vaccine.
Is it safe to conduct Phase III trials?
Just after the Chinese government allowed R&D companies to urgently vaccinate several vaccines in Phase III trials, the Chinese people also had heated discussions about the safety and effectiveness of the vaccines.
Some Chinese citizens said that the effective control of the epidemic made them unnecessary for vaccination for the time being, but there were also some international students like Wu Yilu who did their best to get a place for trial vaccination.
Tinsley Zhang, a Chinese student majoring in media and marketing in the UK, told ABC that she had received two doses of the COVID-19 vaccine developed by Sinopharm at the same time a week before returning to Newcastle.
She said that previous vaccine scandals in China made it difficult for her to choose. However, she decided to accept the possible risks of the vaccine, because the epidemic in the UK worried her and her family more than the safety of the trial vaccine.
“I was very happy when I went to get the vaccine. The process of vaccination is no different from other vaccines,” Tinsley said.
Dr. Mary-Louise McLaws is a member of the advisory group for a COVID-19 epidemic and health emergency project established by the World Health Organization (WHO) and is a professor at the University of New South Wales (UNSW) in Australia.
Dr. McLaws said that the Chinese government’s provision of Phase III vaccines to citizens “is not against ethics” because the students’ decision to vaccinate is “voluntary”.
“Sometimes there are undesirable reactions that scientists didn’t even think of,” said Dr. McLauss.
“They still need to perform blood tests on a regular basis… Make sure that the pharmaceutical companies track them to ensure that they are producing antibodies and that the number of antibodies is high.
“These international students also need to wear masks in public and try to keep their distance even if they are vaccinated.”
Vaccines will not end masks and social distancing
With the declining number of active cases in Australia, people are returning to social life one after another, welcoming summer at beaches and bars.
However, Dr. Maclaus warned that even after the successful development of a vaccine, we are still a long way from returning to normal life. She explained that a successful vaccine does not mean that it can protect everyone.
Today, WHO has urged countries to prepare so that they can start production as soon as a successful vaccine is developed.
Dr. McLaws said that China has the capacity to produce hundreds of millions of vaccines, but she pointed out that every person needs at least two vaccines, so the production of vaccines cannot be directly interpreted as the number of people vaccinated.
“Many vaccines fail in the third stage, and many vaccines fail when they are approved, often because they did not achieve the main result, which is safety, and of course [whether] cause an immune response,” she said.
“However, given the current special circumstances, vaccines with less than 70% of subjects’ immune responses may also be approved.
Wu Yilu plans to return to the UK in January next year. But she plans to continue wearing a mask and follow social distancing measures until her life is free from the impact of the new coronavirus.
“I am doing my best to prepare for the worst,” she said.