A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
In the history of human medicine, only two vaccines have been proven to directly prevent cancer, both targeting cancers caused by specific viruses: the hepatitis B vaccine prevents liver cancer caused by the hepatitis B virus, and the HPV vaccine prevents cervical cancer caused by human papillomavirus.
Lung cancer has the highest incidence and mortality rates globally among cancers. Imagine if one day, with just one injection, we could prevent 90% of lung cancer cases. That would be truly remarkable. And this seemingly distant dream is about to become a reality.
LungVax: A New Dawn for Cancer Prevention
According to information released by the University of Oxford on March 22nd, researchers from the University of Oxford, the Francis Crick Institute, and University College London have received £1.7 million (approximately 2.15 million US Dollar) in funding to develop the world’s first preventive lung cancer vaccine.
Named LungVax, this vaccine is not only used to treat lung cancer but, more impressively, can prevent the formation of lung cancer. Currently in the laboratory development stage, the research team is validating its ability to successfully and stably trigger the body’s immune response.
Sarah Blagden, founder of the LungVax project and professor of experimental oncology at the University of Oxford, emphasized the vaccine’s “the earlier, the better” preventive nature. She stated that stopping the formation of lung cancer in high-risk populations with a vaccine is a crucial step in preventing this devastating disease. According to computer models and preliminary studies, LungVax has the potential to cover approximately 90% of lung cancer types.
The funding for this stage of development comes from Cancer Research UK and the CRIS Cancer Foundation. They plan to invest funds over the next two years to advance research and complete the initial production of the first batch of 3,000 doses of the vaccine.
Michelle Mitchell, CEO of Cancer Research UK, is confident about LungVax’s prospects. She believes that following the success of COVID-19 vaccine development, LungVax should be able to make rapid progress in cancer prevention. The technology used in LungVax is similar to that used in the successful Oxford/AstraZeneca COVID-19 vaccine.
The Mystery of Cancer Prevention: Identifying Lung Cancer’s “ID Card”
COVID-19 is a viral infection, while cancer is a complex disease with various causes. How is the vaccine technology used for COVID-19 applied to cancer? The answer lies in their mechanisms of action.
When our bodies encounter the COVID-19 virus, the vaccine generates an immune response by recognizing the spike protein on the virus. The Oxford-developed COVID-19 vaccine is an adenovirus vector vaccine, which carries the DNA for producing the spike protein inside a harmless adenovirus vector. When this vector enters the body, our cells start expressing the spike protein, tricking our immune system into believing it’s infected with the COVID-19 virus, thus generating an immune response that provides protection.
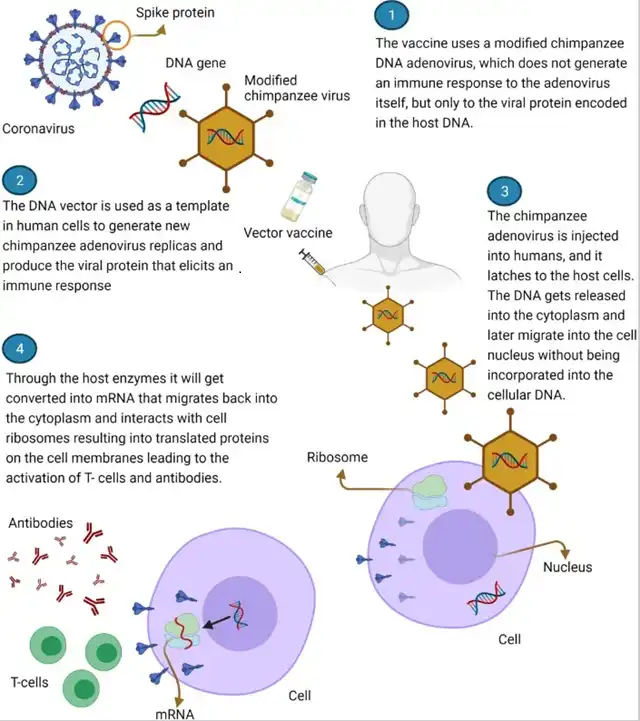
How vaccines work Image source: Reference 2
Unlike viral infections, cancer cells are mutations of our own tissue cells, not foreign invaders like viruses or bacteria. This presents a challenge to the immune system: how to distinguish between normal cells and cancer cells, and only target the cancer cells for attack?
This is the problem that the preventive lung cancer vaccine LungVax aims to solve. The key to solving this problem is for the vaccine to find a molecule that is expressed on lung cancer cells but not on normal cells, acting as a “label” for lung cancer cells, allowing the immune system to accurately recognize them.
Through in-depth research on lung cancer cells, the research team has identified such a protein, which they have named “neoantigen.” This antigen is like an “ID card” for lung cancer cells, unique to lung cancer cells only. The vaccine’s task is to present this antigen to the immune system, teaching it to recognize and attack lung cancer cells.
This is the mechanism of action of the LungVax vaccine: using the traditional adenovirus vector vaccine mechanism, introducing the DNA of the cancer cell neoantigen into the body, allowing the immune system to pre-learn the “special appearance” of cancer cells. This way, when cancer cells do appear, the immune system can kill them off like it does with foreign viruses, effectively eliminating cancer in its early stages.
The Past and Present of Cancer Vaccines: What Choices Do We Have Now?
Since the late 19th century, scientists have been exploring the relationship between cancer and the immune system. It wasn’t until the end of the 20th century that the idea of cancer vaccines truly took shape. During this period, as immunotherapy developed and cancer immunology research deepened, people gradually realized that activating the body’s immune system to recognize and attack cancer cells was possible. This is also the principle behind modern therapies such as immune checkpoint inhibitors.
Development of Preventive Vaccines
As early as 2010, the autologous immune cell therapy sipuleucel-T became the first FDA-approved vaccine therapy for solid malignant tumors (prostate cancer). Studies at the time showed that sipuleucel-T could extend survival by 4.1 months. However, due to its high cost and complex manufacturing process, the vaccine did not see widespread use. Additionally, phase III studies of PSA-based cancer vaccines, such as PROSTVAC-VF for prostate cancer, did not produce satisfactory results.
By 2013, a series of phase III clinical trials of cancer vaccines targeting tumor-associated antigens (TAAs) failed to achieve the expected efficacy. A key issue with TAAs is that they are not unique to tumor cells. Although these antigens are significantly increased in tumor cells, normal cells can also produce these antigens at lower levels. This characteristic poses certain risks in terms of immune activation and safety for vaccines based on TAAs.
Progress in Therapeutic Vaccines
In the same year, with the confirmation of the immune response to new antigens in cancer cells, therapeutic cancer vaccines were recognized as the most influential scientific advancement of the year by the journal “Science.”
Subsequently, in 2017, mRNA cancer vaccines for personalized treatment entered human trials for the first time. Since then, the development of therapeutic cancer vaccines has adopted a standardized process: obtaining tumor samples from patients, identifying and selecting targeted new antigens through gene sequencing, and designing vaccines based on this information. This approach has not only promoted the optimization of vaccine formulations but also provided new ideas for drug administration methods and combination with other treatment methods.
Looking at LungVax in comparison, it is more like a combination of traditional adenovirus vectors and virus vaccines as a preventive vaccine. Moreover, this ready-made vaccine based on Oxford vaccine technology has already proven its effectiveness in preventing diseases during the COVID-19 pandemic. If researchers can replicate the success seen in trials during the pandemic, the immune system could kill cancer in its early stages like it does with foreign viruses. This could save a huge number of lives every year.
In Conclusion
Humanity has been tirelessly pursuing ways to prevent and treat cancer, and each scientific advancement has opened up new possibilities. It is believed that the LungVax vaccine will bring us more good news in the near future, bringing us closer to a future where we can effectively control or even eliminate the threat of cancer.
A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
References:
[1]https://www.ox.ac.uk/news/2024-03-22-new-funding-development-worlds-first-lung-cancer-vaccine
[2]Mascellino MT, Di Timoteo F, De Angelis M, Oliva A. Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect Drug Resist. 2021 Aug 31;14:3459-3476. doi: 10.2147/IDR.S315727.
[3]Biswas N, Chakrabarti S, Padul V, Jones LD, Ashili S. Designing neoantigen cancer vaccines, trials, and outcomes. Front Immunol. 2023 Feb 9;14:1105420. doi: 10.3389/fimmu.2023.1105420.
[4]Korman, A.J., Garrett-Thomson, S.C. & Lonberg, N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov 21, 509–528 (2022). https://doi.org/10.1038/s41573-021-00345-8
[5]https://www.fda.gov/media/78511/download
[6]Fan T, Zhang M, Yang J, Zhu Z, Cao W, Dong C. Therapeutic cancer vaccines: advancements, challenges, and prospects. Signal Transduct Target Ther. 2023 Dec 13;8(1):450. doi: 10.1038/s41392-023-01674-3. PMID: 38086815; PMCID: PMC10716479.
[7]https://www.science.org/content/article/sciences-top-10-breakthroughs-2013
[8]Deng Z, Tian Y, Song J, An G, Yang P. mRNA Vaccines: The Dawn of a New Era of Cancer Immunotherapy. Front Immunol. 2022 Jun 2;13:887125. doi: 10.3389/fimmu.2022.887125. PMID: 35720301; PMCID: PMC9201022.
[9]Xie W, Chen B,Wong J. Evolution of the market for mRNA technology[J]. Nature reviews. Drugdiscovery, 2021, 20(10): 735-736.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



