Five immune microenvironmental subtypes of liver cancer defined for the first time at single-cell precision
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Nature: Five immune microenvironmental subtypes of liver cancer defined for the first time at single-cell precision
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Nature: Five immune microenvironmental subtypes of liver cancer defined for the first time at single-cell precision.
The heterogeneity of the immune microenvironment is one of the important reasons for tumor drug resistance, recurrence and poor prognosis.
In recent years, immunotherapy and combination therapy have brought hope to patients with advanced tumors.
Systematic exploration of the heterogeneity of the tumor immune microenvironment plays an important role in guiding treatment selection, efficacy prediction, program optimization, and the development of new immunotherapy targets.
Zhang Ning ‘s team at Peking University First Hospital has been focusing on the exploration of liver cancer heterogeneity in recent years.
Through a series of preliminary work, it has revealed the genomic heterogeneity ( Gastroenterology , 2016) , phenotypic heterogeneity (Cancer Cell, 2019) and Single-cell copy number heterogeneity (Gastroenterology, 2022) .
However, there is still a lack of systematic and in-depth exploration of the heterogeneity of the immune microenvironment in liver cancer.
Previous studies were mainly based on pathological analysis or Bulk transcriptome sequencing, and the results could not accurately analyze the cell composition and easily overlooked the key cell subpopulations with less content.
The development of single-cell sequencing technology has promoted the exploration in the field of tumor immunity.
At present, many studies have conducted single-cell level exploration of liver cancer.
However, most of these studies focus on specific types of cells, and the results cannot reflect the full characteristics of the immune microenvironment.
Therefore, an unbiased research strategy that includes all cell subtypes is urgently needed to systematically reveal the heterogeneity of the immune microenvironment in HCC.
On November 10, 2022, researchers from the Cancer Translational Research Center of Peking University First Hospital worked closely with researchers from Peking University’s Biomedical Frontier Innovation Center (BIOPIC) and the Department of Hepatobiliary Surgery at Peking University People’s Hospital to publish a paper in Nature entitled : Research paper on Liver tumor immune microenvironment subtypes and neutrophil heterogeneity .
This study defined five immune microenvironmental subtypes of liver cancer at single-cell precision for the first time , and named it the TIMELASER typing system. The tumor-promoting mechanism of the two key subgroups of L1 + TAN finally proved that targeting tumor-associated neutrophils is expected to form a new tumor-promoting mechanism through the construction of a mouse liver cancer model from the three levels of In vitro, Ex vivo, and In vivo. Liver cancer immunotherapy program, these achievements provide key information for basic research and clinical diagnosis and treatment of liver cancer.

In order to reveal the characteristics of the tumor microenvironment of liver cancer, the researchers completed single-cell transcriptome sequencing without antibody enrichment of a total of 189 samples from humans and mice (Figure 1) .
At the same time, the researchers performed exome sequencing on the supporting cases, and collected 8 published single-cell transcriptome sequencing data sets, 453 tissue transcriptome sequencing and 10 spatial transcriptome data for validation analysis, and comprehensively utilized tumor An In vitro culture system was established for cell lines , an Ex vivo experimental system was constructed based on clinical samples , and functional verification was performed based on an In vivo experimental system based on a mouse tumor model.
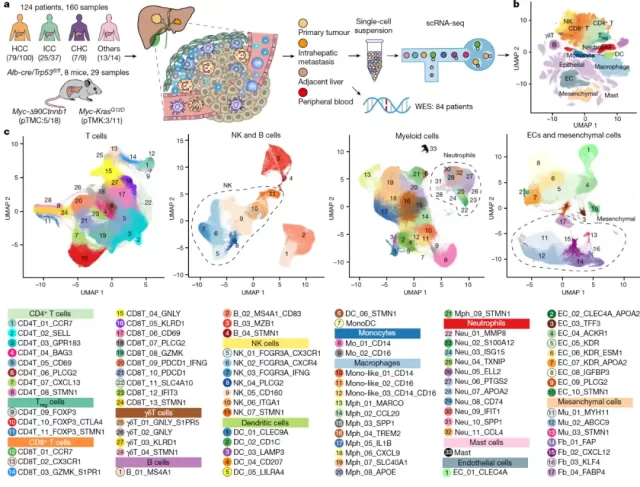 Figure 1: Experimental design and cell subpopulations discovered in this study
Figure 1: Experimental design and cell subpopulations discovered in this study
Five immune microenvironmental subtypes of liver cancer
First, the researchers systematically analyzed 89 cell subsets in the immune microenvironment of liver cancer. Through hierarchical clustering analysis, the researchers successfully resolved 5 different immune microenvironment subtypes, including:
① immune activation type TIME-IA (Immune Activation);
② myeloid- enriched immunosuppressive type TIME-ISM (Immune Suppressive Myeloid) ;
③ matrix-enriched immunosuppressive type TIME-ISS (Immune Suppressive Stromal) ;
④ immune rejection type TIME-IE (Immune Exclusion) ;
⑤ immune resident type (Immune Residence) , combined called TIMELASER typing system (Figure 2) .
Subsequently, the researchers conducted a multidimensional analysis of the five immune microenvironment subtypes, and verified the existence of the five subtypes through large-scale transcriptome data; revealed the five subtypes through spatial transcriptome data and CODEX multicolor immune technology The spatial distribution of cells in different subtypes; through the analysis of receptor ligands, it is found that there are different chemokine receptor-ligand networks in each subtype, suggesting the formation mechanism of different subtypes; combined with exome data analysis, it is found that different subtypes Different driver gene mutations were enriched, such as TP53 , CTNNB1 , KRAS and IDH1 , and different subtypes were found to enrich different tumor cell gene modules. The discovery of these five immune microenvironments provides important reference information for tumor immunotherapy.
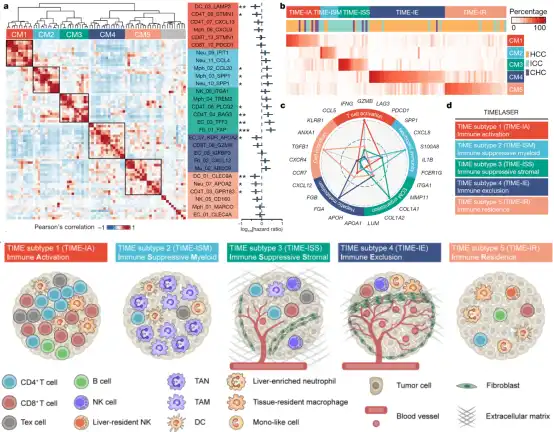
Figure 2: Schematic diagram of the five subtypes of the tumor immune microenvironment (TIMELASER)
Functional heterogeneity of tumor-associated neutrophils
Neutrophils are a type of very fragile cells. It is generally believed that they can survive for no more than one week after entering the peripheral blood in vivo, and no more than 24 hours in vitro.
Therefore, none of the previous single-cell studies of liver cancer had captured this population of cells.
Thanks to the rapid experimental process and antibody-free enrichment strategy, more than 30,000 neutrophils were successfully captured in this study.
After cluster analysis, the researchers found a total of 11 neutrophil subgroups, which were enriched in peripheral blood, paracancerous and tumor tissues respectively (Figure 3) , and successfully identified 6 groups of tumor-associated neutrophils.
Subsequently, the researchers analyzed the developmental trajectories and key transcription factors of these neutrophil subsets, and found that two neutrophil subsets, CCL4 + TAN and PD-L1 + TAN, may promote tumor growth through different mechanisms.
In order to further verify these findings, the researchers constructed liver cancer cell line-neutrophil in vitro co-culture system ( In vitro ) and liver cancer patient neutrophil in vitro analysis system ( Ex vivo ) , through transcriptome sequencing, ATAC- Experiments such as seq and multicolor immunofluorescence verified that CCL4 + TAN promotes tumor growth by recruiting tumor-associated macrophages, while PD-L1 + TAN promotes tumor growth by inhibiting the killing function of CD8 + T cells.
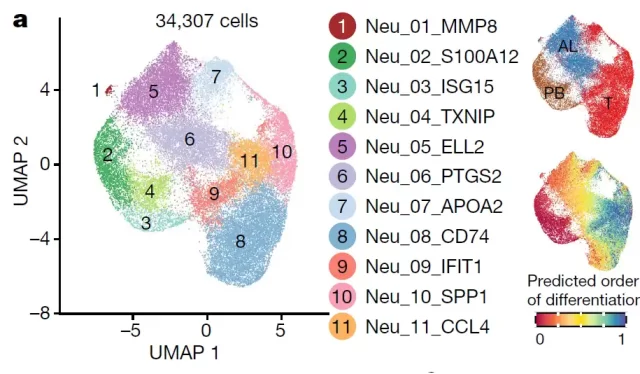 Figure 3: Tissue distribution and developmental trajectories of neutrophil subsets
Figure 3: Tissue distribution and developmental trajectories of neutrophil subsets
A mouse liver cancer model was constructed, revealing that its neutrophil subsets are highly conserved with humans, and confirming that removing neutrophils can delay tumor growth
In order to further explore the tumor – promoting mechanism of neutrophils in vivo, the researchers constructed two mouse liver cancer spontaneous tumor models ( Myc -∆90Ctnnb1 , pTMC; Myc-Kras G12D , pTMK) , and single-cell sequencing analysis.
The researchers found that the neutrophil subsets in mice are highly conserved with humans, which indicates that experiments using mouse liver cancer models can provide important reference information for the treatment of human liver cancer.
Subsequently, the researchers used the Anti-Ly6G antibody to conduct neutrophil depletion experiments in mouse models, and the results showed that after treatment, the growth of liver cancer in mice was effectively curbed (Figure 4) .
Further, the researchers also revealed the dynamic changes of neutrophil subsets in mouse bone marrow, peripheral blood, paracancerous and tumor tissues during treatment. These results suggest that the development of neutrophil-based immunotherapy targets is expected to form a new treatment strategy for liver cancer.
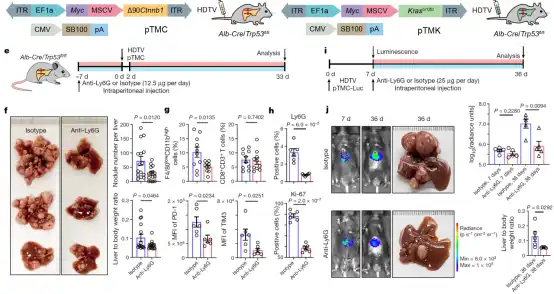
Figure 4: Anti-Ly6G antibody depletion of neutrophils can effectively alleviate the growth of liver cancer in mice
In summary, this study systematically revealed the immune microenvironment subtypes of liver cancer, and deeply analyzed the functional heterogeneity of tumor-associated neutrophils.
Finally, the mouse liver cancer model proved that targeting tumor-associated neutrophils is expected to form a new immune system. Immunotherapy strategies for liver cancer.
The research results indicate that the intervention of tumor-associated neutrophils is expected to greatly increase the patient population for effective treatment of immune checkpoints.
These results provide key information for basic research and clinical diagnosis and treatment of liver cancer and even solid tumors.
Paper link :
https://www.nature.com/articles/s41586-022-05400-x
Nature: Five immune microenvironmental subtypes of liver cancer defined for the first time at single-cell precision
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



