The world first β-amyloid antibody officially approved for the treatment of Alzheimer’s disease
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The world first β-amyloid antibody officially approved for the treatment of Alzheimer’s disease
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The world first β-amyloid antibody officially approved for the treatment of Alzheimer’s disease .
On July 07, The FDA officially approved Lecanemab (BAN2401) for the treatment of Alzheimer’s disease (AD) in adults [1]. Lecanemab was qualified for accelerated approval in January this year.
Based on the results of the Clarity AD (study 301) study, the FDA converted the accelerated approval to a formal approval. This is the first Aβ antibody drug officially approved for the treatment of Alzheimer’s disease.

Lecanemab (BAN2401) is a humanized IgG1 antibody that selectively binds to larger soluble Aβ fibrils. A researcher once compared the binding abilities of Lecanemab, Aducanumab and Gantenerumab to various forms of Aβ polymers horizontally.
The results showed that the affinity of Lecanemab for binding 75-300kD smaller fibrils was 10 times that of Gantenerumab and 100 times that of Aducanumab; for 300- For larger fibrils of 500kD, the affinity of Lecanemab is 25 times that of Aducanumab [2].
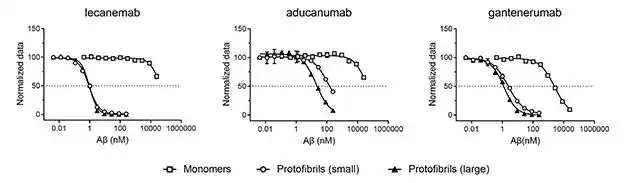 Lecanemab primarily binds to Aβ fibrils
Lecanemab primarily binds to Aβ fibrils
On November 29, 2022, Eisai announced the results of the phase 3 clinical study Clarity AD of lecanemab at the CATD conference held in San Francisco, and the paper was simultaneously published in the New England Journal of Medicine (NEJM).
Clarity AD is an 18-month, multicenter, double-blind, Phase 3 clinical trial enrolling 1,795 patients with early AD, defined as AD-associated mild cognitive impairment confirmed by positron emission tomography (PET) or cerebrospinal fluid evidence or patients with mild dementia.
Participants were randomly assigned to treatment group and placebo group, received 10mg/kg/biweekly intravenous injection treatment, the primary endpoint was the change in CDR-SB score from baseline, and the secondary endpoints were changes in amyloid burden detected by PET and ADAS-cog14 , ADCOMS, ADCS-MCI-ADL scale score changes.
The baseline CDR-SB scores of both groups were 3.2, which met the standard range of early AD (0.5-6).
At 18 months, the CDR-SB score was 1.21 in the treatment group and 1.66 in the placebo group, with an absolute difference of -0.45, and the score decline was delayed by 27%.
Three other cognitive/behavioral assessments also showed significant improvements with lecanemab.
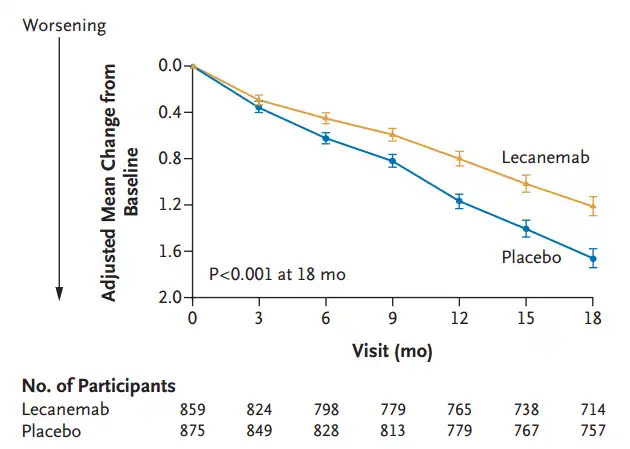 The decline of CDR-SB score in the treatment group was significantly delayed
The decline of CDR-SB score in the treatment group was significantly delayed
The safety profile of lecanemab is relatively good. Infusion-related reactions occurred in 26.4% of patients in the treatment group, and ARIA-E occurred in 12.6%. 91% of ARIA-E were mild to moderate, 78% were asymptomatic, 71% occurred within the first three months of treatment, and 81% resolved within 4 months of occurrence.
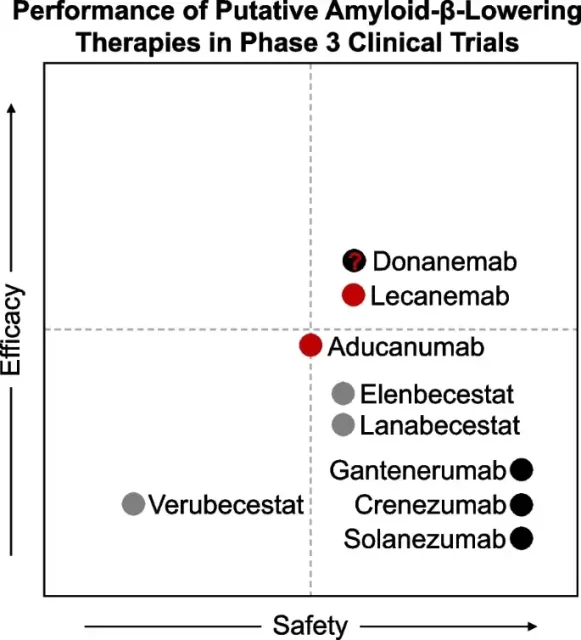
In a review article recently published in BMC Molecular Neurodegenerative Diseases [3], the safety and effectiveness of some new AD drugs that have received more attention were compared.
It can be seen that Lecanemab is relatively good. The review also pointed out that the efficacy of lecanemab in Clarity AD is basically equivalent to a delay of 4-5 months in disease-related progression during treatment.
Eisai, the developer of Lecanemab, issued a statement [4] today, announcing the pricing measures of Lecanemab in the United States.
Based on a biweekly dose of 10 mg/kg, the annual medical cost of the patient is approximately $26,500 based on the average body weight of the subjects in Study 201 and Clarity AD of 75 kg . Considering the packaging capacity of different medicines, this number may fluctuate slightly.
They also pointed out that after significant Aβ clearance, a low-dose maintenance regimen may be adopted, which can further reduce medical costs.
References:
[1] https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval
[2] https://www.alzforum.org/news/conference-coverage/lecanemab-sweeps-toxic-av-protofibrils-catches-eyes-trialists
[3] https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-023-00637-0
[4] https://www.prnewswire.com/news-releases/eisais-approach-to-us-pricing-for-leqembi-lecanemab-a-treatment-for-early-alzheimers-disease-sets-forth-our -concept-of-societal-value-of-medicine-in-relation-to-price-of-medicine-301715694.html
The world first β-amyloid antibody officially approved for the treatment of Alzheimer’s disease
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



