First in 20 years! New drug for Alzheimer’s disease wins unanimous support from FDA
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
First in 20 years! New drug for Alzheimer’s disease wins unanimous support from FDA, laying the groundwork for wider use
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
First in 20 years! New drug for Alzheimer’s disease wins unanimous support from FDA, laying the groundwork for wider use.
On January 6, 2023, the Alzheimer’s disease drug lecanemab (trade name Leqembi) developed by Eisai and Biogen received conditional approval from the US FDA .
Results from its phase 3 clinical trial showed the drug slowed the rate of cognitive decline by 27% in Alzheimer’s patients . The FDA has since conducted a further review of the clinical trial results to determine whether the drug can be fully approved .
On June 10, 2023, at the US FDA Peripheral and Central Nervous System Drugs Advisory Committee meeting, committee experts voted unanimously 6:0 to fully approve lecanemab (trade name Leqembi) .
The FDA is expected to make a final decision by July 6 this year. If fully approved, it will become the first new drug for Alzheimer’s disease to be fully approved by the FDA in 20 years.
The FDA decision will have special significance because the Medicare agency has been delaying approval for lecanemab .
Reimbursement paid until it can be fully approved by the FDA, its treatment costs $26,500 per year.

Lecanemab is also jointly developed by Eisai and Biogen . On November 29, 2022, the Phase III clinical trial results of the drug were published in the New England Journal of Medicine (NEJM ) .
Results from a clinical trial in early-stage Alzheimer’s patients showed the drug slowed cognitive decline by 27 percent in Alzheimer’s patients .

Lecanemab is a humanized IgG1 monoclonal antibody that works by binding to β-amyloid (Aβ) .
These early-stage Alzheimer’s patients received intravenous lecanemab or a placebo every two weeks for 18 months, and the researchers measured their cognitive abilities on an 18-point scale. The results showed that lecanemab delayed the time to disease progression in patients by about 5 months .
Additionally, patients who received lecanemab were 31 percent less likely to progress to the next stage of their disease during the study period.
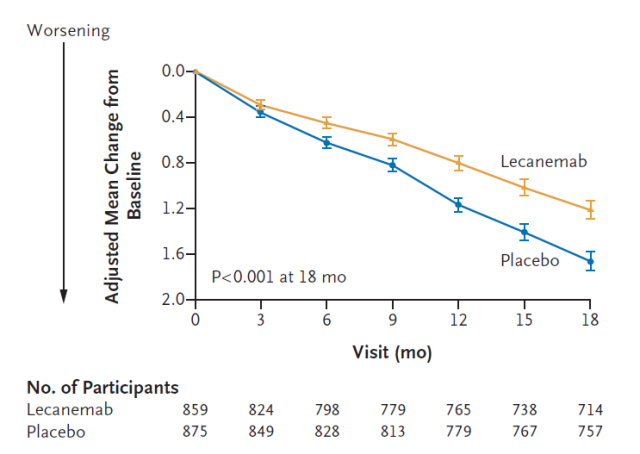
In addition, the adverse reactions in the clinical trial results have caused controversy. 17.3% of patients receiving the drug experienced cerebral hemorrhage (compared with 9% in the placebo control group) , and 12.6% of patients experienced brain swelling (compared with 1.7% in the placebo control group). ) .
In fact, Alzheimer’s disease experts are still divided on how much these changes that the drug brings to Alzheimer’s patients will affect patients and their families.
For example, Dr. Ron Petersen , an Alzheimer’s disease specialist at the Mayo Clinic , believes the drug’s effect is modest, but it’s clinically meaningful. Because even delaying disease progression for a few months can give Alzheimer’s patients a little more time to live/work independently for longer.
Maria Carrillo , chief scientist at the Alzheimer’s Association , said the clinical trial is important because it shows that targeting amyloid with drugs can delay Alzheimer’s disease progression.
Delaying cognitive decline in the early stages of Alzheimer’s disease has important implications, allowing patients to spend more time with loved ones, enjoy family life, travel, vacation, and bucket list.
Drugs that target Aβ can cause side effects including brain swelling and bleeding in the brain, and lecanemab is no exception, and Eisai said most patients had mild or no symptoms.
According to public reports, three Alzheimer’s patients died during clinical studies of the drug, two of whom died from strokes caused by cerebral hemorrhages, which Eisai said were taking blood thinners for other health problems. The deaths could not be attributed to Alzheimer’s drugs.
The chairman of the FDA Peripheral and Central Nervous System Drugs Advisory Committee and Professor Robert Alexander of the University of Arizona said that from the results of clinical trials, lecanemab has side effects, but which side effects can be monitored, and the benefits it brings to patients It is obvious.
References :
https://www.nejm.org/doi/10.1056/NEJMoa2212948
https://medicalxpress.com/news/2023-06-alzheimer-drug-fda-panel-stage.html
First in 20 years! New drug for Alzheimer’s disease wins unanimous support from FDA, laying the groundwork for wider use
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



