Neurofibromatosis type 1 gene mutation promotes growth of optic neuroma
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Nature: Neurofibromatosis type 1 gene mutation promotes the growth of optic neuroma
Neurofibromatosis type 1 gene mutation promotes growth of optic neuroma. The article revealed that neurofibromatosis type 1 gene mutations promote the growth of nerve activity-dependent optic neuroma.
Neurofibromatosis type 1 (NF1) is a genetic disease that can cause brown spots on the skin and abnormal growth of nerves, caused by mutations in the same name gene NF1. The incidence of neurofibromatosis type 1 is about one in four thousand to one in three thousand.
About 15% of neurofibromatosis type 1 (NF1) patients develop poorly differentiated optic neuromas (called optic gliomas, OPG) in early childhood. Recent studies have shown that neurons are essential cellular components of the tumor microenvironment, and their activity can increase tumor growth.
On May 26, 2021, Michelle Monje of the Department of Neurology and Neuroscience of Stanford University and David H. Gutmann of the Department of Neurology of Washington University School of Medicine published an article in the Nature journal revealing that neurofibromatosis type 1 gene mutations promote neural activity dependence Optic neuroma growth.

In order to study the effect of neuronal activity on the growth of low-grade glioma and the formation of glioma, they used a type of Nf1-OPG transgenic mice called poorly differentiated optic neuroma, which has a high tumor incidence. Tumor cells in this model mouse grow on retinal ganglion cells
(RGC) nearby.
The formation of mouse Nf1 optic glioma needs to meet two conditions at the same time: the somatic Nf1 function in neural progenitor cells is lost; the heterozygous Nf1 mutation also exists in non-neoplastic (stromal) cells, similar to NF1 cancer susceptibility syndrome Of patients.
Light-activated RG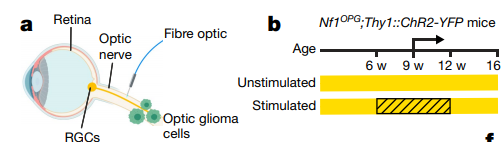 C promotes tumor growth
C promotes tumor growth
They injected optogenetic virus into RGC of Nf1-OPG transgenic mice, and assessed the tumor growth rate at 12 weeks after activating RGC at 6 weeks of age. The results showed that the volume of the optic nerve became larger after light-activated RGC, and the proliferation of glioma cells increased significantly, which indicated that the nerve activity of RGC promoted the growth of optic neuroma.
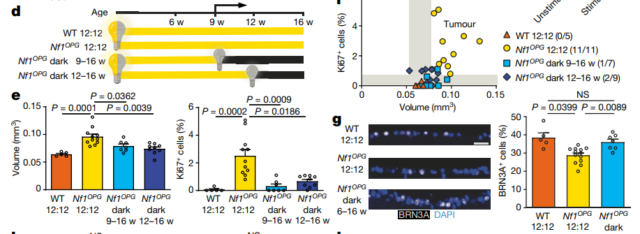
Dark environment inhibits tumor growth
More interestingly, sunlight affects tumor growth in Nf1-OPG transgenic mice: in full light (
The tumor grows fast under the condition of 24 hours of light, but in total darkness (24 hours of darkness)
The tumor grows slowly, and the proliferation of tumor cells decreases. Could it be said that wearing sunglasses to avoid light can inhibit tumor growth?
Previous studies have found that cortical neuronal activity drives the growth of well-differentiated tumors through paracrine BDNF and synaptoadhesin NLGN3. NLGN3 is a cell adhesion protein on the postsynaptic membrane, which can mediate the formation and maintenance of synapses between neurons.
Researchers found that light-activated RGC can indeed cause the levels of BDNF and NLGN3 to increase, and incubating NLGN3 in vitro can also promote the growth of poorly differentiated optic neuroma. In addition, the expression of NLGN3 was up-regulated in patients with Nf1 optic neuroma.
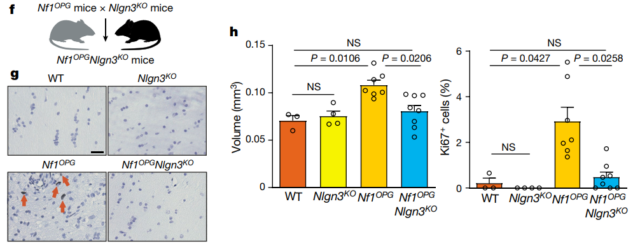
Knockout of NLGN3 inhibits tumor growth
After knocking out NLGN3, the tumor growth of Nf1-OPG transgenic mice seemed to have been paused: the volume of the optic nerve was no different from normal, and the proliferation of tumor cells was significantly reduced. This indicates that NLGN3 is involved in the tumor development process of poorly differentiated optic neuroma.
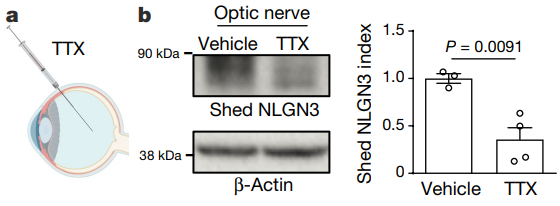
After the researchers injected tetrodotoxin into the vitreous body to inhibit neuronal activity, the expression of NLGN3 secreted by the optic nerve was significantly reduced. Optogenetics technology did not affect the secretion of NLGN3 after activating the optic nerve of normal mice, but NLGN3 expression was up-regulated in NF1 mutant mice.
Completely in the dark environment does not affect the secretion of NLGN3 in normal mice. NLGN3 expression is up-regulated in NF1 mutant mice. These results indicate that Nf1 mutation abnormally increases the up-regulation of NLGN3, which is regulated by neuronal activity in the optic nerve.
Disintegrin and metalloprotease 10 (ADAM10) is a “sheddase” that can hydrolyze more than 30 transmembrane proteins. NLGN3 is shed from the neurons by ADAM10. After treatment with GI254023X (ADAM10 inhibitor), it can prevent the growth of tumors in Nf1-OPG transgenic mice and inhibit the proliferation of tumor cells.
Nature also distributed review articles by Varun Venkataramani and Frank Winkler from the Department of Neurology of the German Cancer Research Center and the University Hospital of Heidelberg, Germany.
They said that this is an exciting study. This article uses a tumor gene model mouse to reveal the discovery that light-induced neuronal activity not only promotes tumor growth, but is also responsible for the tumor initiation process. Gene knocking out NLGN3 or inhibiting NLGN3 can slow the growth of tumors. What’s more interesting is that tumor growth slows down in the dark.
So, what is the clinical significance of this research? Should NF1 patients be told that they need to wear sunglasses or cover their eyes? Should somehow reduce the overall neuronal activity of cancer patients?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



