CDKN2A/2B gene mutation organoid pharmacological model
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
CDKN2A/2B gene mutation organoid pharmacological model
CDKN2A/2B gene mutation organoid pharmacological model. It is generally believed that CDKN2A/2B gene loss-of-function mutations may cause abnormal activation of CDK2/4/6, so CDK inhibitors may be used as a potential treatment for patients with CDKN2A/2B mutations.
CDKN2A/2B are tumor suppressor genes, both located on chromosome 9, encoding p16CDKN2A and p15INK4B proteins respectively, which can inhibit the activity of CDK kinase and regulate the G1 cell cycle. The inactivation of CDKN2A/2B may lead to uncontrolled cell growth and proliferation, leading to cancer [1]. (Picture 1 / Picture 2)
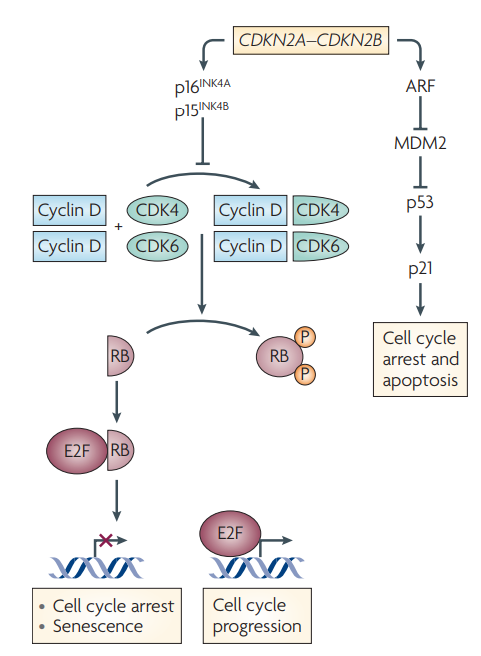
Figure 1. CDKN2A/2B regulates the cell cycle (AleaA. Mills, Nature Review Cancer, 2010)
CDKN2A/2B is the most common somatic mutation gene in metastatic tumors. Deletions, mutations or hypermethylation often occur in many tumors, including head and neck tumors, non-small cell lung cancer, prostate cancer, glioma, esophageal squamous cell carcinoma, Bladder cancer and T-cell lymphoma, among which CDKN2A is considered to be the second most frequently inactivated gene in cancer after p53.
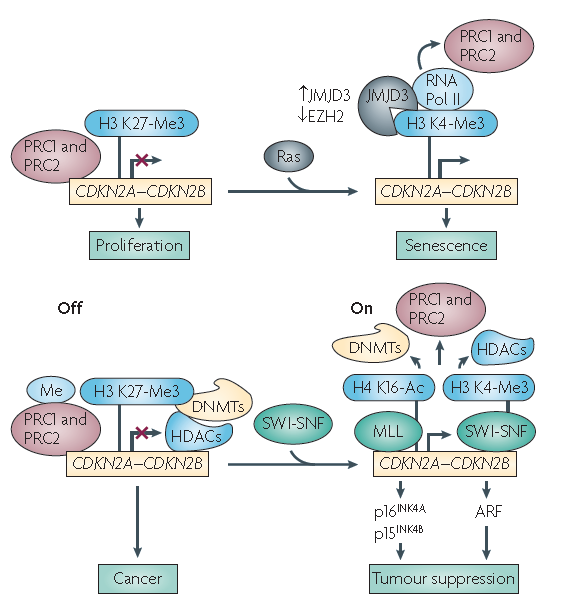
Figure 2. Epigenetic regulation of CDKN2A/2B expression (AleaA. Mills, Nature Review Cancer, 2010)
It is generally believed that CDKN2A/2B gene loss-of-function mutations may cause abnormal activation of CDK2/4/6, so CDK inhibitors may be used as a potential treatment for patients with CDKN2A/2B mutations. One study based on melanoma cell lines supports this hypothesis. The results of the study indicate that CDKN2A methylation, mutation or p16(INK)(4A) protein loss is associated with increased sensitivity to CDK4/6 inhibitor Palbociclib [2]. But in another study, for patients with imatinib/sunitinib-resistant gastrointestinal stromal tumors (GIST) who lacked P16/CDKN2A, pabocillin monotherapy was not effective [3].
Pabocillin has been approved for marketing in ER-positive Her2-negative breast cancer, and its application in other tumor indications, especially the study of co-administration, is becoming a hot spot. Therefore, the development of CDKN2A/2B gene mutation therapy drugs and the study of co-administration have always been a hot spot for medical researchers.
At present, Ketu Medical has successfully constructed a tumor organoid model containing a variety of driving target mutations and somatic mutations by simulating the tumor growth microenvironment. The series of products including CDKN2A/2B gene mutations are as follows (see Table 1):
Table 1 Information on CDKN2A/2B gene mutation model of Ketu Medicine
| CAT# | Clinical case diagnosis | CDKN2A/2B Mutation | Other Pathogenic Mutation |
| KO-73325 | Pancreatic cancer lung metastasis | CDKN2A H83R | KRAS G12D /TP53 c.241dup p.T81Nfs*68/ALK-HEPACAM2 Fusion |
| KO-59933 | Lung cancer | CDKN2A G45R | FGFR4 amplification |
| KO-58075 | Esophageal cancer | CDKN2A V115L | TP53 R249G / TP53 G245D |
| KO-65478 | Stomach cancer | CDKN2A R80* | XPO1 E571K / XPO1 E571K |
| KO-41723 | Bladder Cancer | CDKN2A/CDKN2B Deletion | SPTA1 .G505R/PIK3CA E545K/FGFR3 Y373C/FOXO1 P519L/MED12 G44D |
| KO-46617 | Pancreatic cancer | CDKN2A/CDKN2B Deletion | KRAS G12V/TP53 V203E |
| KO-4306 0 | Esophageal cancer | CDKN2A/CDKN2B Deletion | PIK3CA Q546K/ STK11 P294L/ |
| KO-96253 | Lung cancer | CDKN2A/CDKN2B Deletion | EGFR L858R/ EGFR /MET/MYC/CCND1/CDK6 amplification |
Related Services
- Organoid model construction service
- Organoid model identification related testing services
- Cell drug efficacy testing service
- Drug synergy testing service
- PDOX mouse model in vivo drug efficacy testing service
(source:internet, reference only)
Disclaimer of medicaltrend.org



