Insights from Terminated ADCs: Glembatumumab Vedotin and Depatuxizumab Mafodotin
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Insights from Terminated ADCs: Glembatumumab Vedotin and Depatuxizumab Mafodotin
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Insights from Terminated ADCs: Glembatumumab Vedotin and Depatuxizumab Mafodotin
Over 260 antibody-drug conjugates (ADCs) have been studied in clinical trials for various cancer indications, with 92 of them terminated for reasons such as intolerable toxicity, insufficient efficacy, or commercial considerations.
In this analysis, we explore the data and reasons for termination of two ADCs: Glembatumumab Vedotin (CDX-011) and Depatuxizumab Mafodotin (ABT-414).
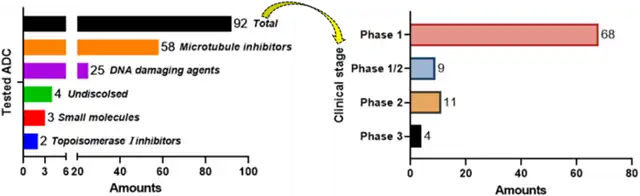
Figure 1. Brief overview of ADCs that have terminated clinical studies (classified by payload and clinical progress)
Glembatumumab Vedotin (CDX-011)
Developed by Celldex Therapeutics from 2006 to 2018, CDX-011 aimed to treat non-metastatic melanoma protein B (GPNMB)-expressing cancers. Despite promising preclinical data, the Phase III trial was discontinued on April 18, 2018, as CDX-011 failed to demonstrate superior efficacy over Xeloda® in metastatic triple-negative breast cancer (high GPNMB expression).
Preclinical Studies
CDX-011, a fully humanized IgG2 monoclonal antibody, showed effective and specific inhibition of GPNMB+ melanoma cells when conjugated with monomethyl auristatin E (MMAE). In mouse xenograft models, CDX-011 demonstrated dose-dependent tumor growth inhibition, with some tumors completely regressing.
Clinical Trials and Termination
Initiated in June 2006, CDX-011 progressed through Phase I, II, and III trials. However, the Phase III results did not meet the primary endpoint for metastatic triple-negative breast cancer. Common Grade ≥3 adverse events included severe rash, and the termination of the CDX-011 development plan led to a 20% workforce reduction at Celldex. Subsequent ADC development (CDX-014) targeting TIM-1 was also canceled.
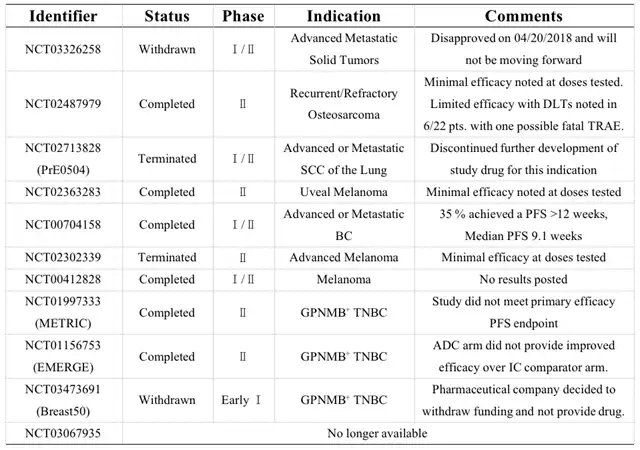
Depatuxizumab Mafodotin (ABT-414)
Developed by Abbvie from 2013 to 2019, ABT-414 targeted the epidermal growth factor receptor (EGFR) and focused on treating glioblastomas.
Preclinical Studies
ABT-414, composed of ABT-806 and MMAF, demonstrated low normal tissue binding and potent cytotoxicity against EGFR-expressing tumor cell lines in preclinical studies. In xenograft models, ABT-414 showed significant tumor growth inhibition and regression, particularly in models expressing wild-type or mutated EGFR.
Clinical Trials and Termination
Despite promising preclinical results, ABT-414 faced challenges in clinical trials. Although granted orphan drug designation for glioblastoma treatment, a Phase III trial (UNITE) combining ABT-414 with radiochemotherapy was terminated in 2019 due to lack of survival benefit. Notably, ABT-414 exhibited some ocular toxicity.
Conclusion
While both ADCs showed promise in preclinical studies, the translation to clinical success proved challenging.
Lessons from these terminations highlight the complexity of ADC development, emphasizing the importance of rigorous clinical evaluation and the need for a deeper understanding of factors influencing efficacy and toxicity.
Insights from Terminated ADCs: Glembatumumab Vedotin and Depatuxizumab Mafodotin
References:
1. Maecker, H., et al., Exploration of the antibody–drug conjugate clinical landscape. mAbs, 2023. 15(1): p. 2229101.
2. Foltz, I.N., K. Gunasekaran, and C.T. King, Discovery and bio-optimization of human antibody therapeutics using the XenoMouse® transgenic mouse platform. Immunol Rev, 2016. 270(1): p. 51-64.
3. Tse, K.F., et al., CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clinical Cancer Research, 2006. 12(4): p. 1373-1382.
4. Doronina, S.O., et al., Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol, 2003. 21(7): p. 778-84.
5. Naumovski, L. and J.R. Junutula, Glembatumumab vedotin, a conjugate of an anti-glycoprotein non-metastatic melanoma protein B mAb and monomethyl auristatin E for the treatment of melanoma and breast cancer. Curr Opin Mol Ther, 2010. 12(2): p. 248-57.
6. Pollack, V.A., et al., Treatment parameters modulating regression of human melanoma xenografts by an antibody–drug conjugate (CR011-vcMMAE) targeting GPNMB. Cancer Chemotherapy and Pharmacology, 2007. 60(3): p. 423-435.
7. https://ir.celldex.com/news-releases/news-release-details/celldexs-metric-study-metastatic-triple-negative-breast-cancer-0.
8. Vaklavas, C. and A. Forero, Management of metastatic breast cancer with second-generation antibody-drug conjugates: focus on glembatumumab vedotin (CDX-011, CR011-vcMMAE). BioDrugs, 2014. 28(3): p. 253-63.
9. Hasanov, M., et al., A Phase II Study of Glembatumumab Vedotin for Metastatic Uveal Melanoma. Cancers, 2020. 12(8): p. 2270.
10. Ott, P.A., et al., A phase 2 study of glembatumumab vedotin, an antibody-drug conjugate targeting glycoprotein NMB, in patients with advanced melanoma. Cancer, 2019. 125(7): p. 1113-1123.
11. Vahdat, L.T., et al., Glembatumumab vedotin for patients with metastatic, gpNMB overexpressing, triple-negative breast cancer (“METRIC”): a randomized multicenter study. npj Breast Cancer, 2021. 7(1): p. 57.
12. Yardley, D.A., et al., EMERGE: A Randomized Phase II Study of the Antibody-Drug Conjugate Glembatumumab Vedotin in Advanced Glycoprotein NMB–Expressing Breast Cancer. Journal of Clinical Oncology, 2015. 33(14): p. 1609-1619.
13. Kopp, L.M., et al., Phase II trial of the glycoprotein non-metastatic B-targeted antibody–drug conjugate, glembatumumab vedotin (CDX-011), in recurrent osteosarcoma AOST1521: A report from the Children’s Oncology Group. European Journal of Cancer, 2019. 121: p. 177-183.
14. https://classic.clinicaltrials.gov/ct2/show/NCT02837991.
15. Phillips, A.C., et al., ABT-414, an Antibody–Drug Conjugate Targeting a Tumor-Selective EGFR Epitope. Molecular Cancer Therapeutics, 2016. 15(4): p. 661-669.
16. Reilly, E.B., et al., Characterization of ABT-806, a Humanized Tumor-Specific Anti-EGFR Monoclonal Antibody. Mol Cancer Ther, 2015. 14(5): p. 1141-51.
17. Cleary, J.M., et al., A phase 1 study of ABT-806 in subjects with advanced solid tumors. Invest New Drugs, 2015. 33(3): p. 671-8.
18. Lassman, A.B., et al., Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: A phase III randomized clinical trial. Neuro-Oncology, 2022. 25(2): p. 339-350.
19. Reardon, D.A., et al., Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro Oncol, 2017. 19(7): p. 965-975.
20. Parrozzani, R., et al., Corneal side effects induced by EGFR-inhibitor antibody-drug conjugate ABT-414 in patients with recurrent glioblastoma: a prospective clinical and confocal microscopy study. Ther Adv Med Oncol, 2020. 12: p. 1758835920907543.
21. Parrozzani, R., et al., Ocular Side Effects of EGFR-Inhibitor ABT-414 in Recurrent Glioblastoma: A Long-Term Safety Study. Front Oncol, 2020. 10: p. 593461.
22. Naseri Kouzehgarani, G., et al., Biodistribution Analysis of an Anti-EGFR Antibody in the Rat Brain: Validation of CSF Microcirculation as a Viable Pathway to Circumvent the Blood-Brain Barrier for Drug Delivery. Pharmaceutics, 2022. 14(7): p. 1441.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



