More Than Just Cancer: ADCs Find Applications Across Various Disease Areas
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
More Than Just Cancer: ADCs Find Applications Across Various Disease Areas
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
More Than Just Cancer: ADCs Find Applications Across Various Disease Areas
When it comes to Antibody-Drug Conjugates (ADCs), the immediate association is often with cancer treatment, particularly solid tumors.
Indeed, the majority of ADCs developed to date have primarily focused on treating various types of cancers. However, there’s a growing presence of ADC development for other indications in preclinical studies.
In cancer-related diseases (Figure 1A), research on breast cancer, lymphoma, and hematologic malignancies has been predominant. This aligns with the ADCs approved for cancer treatment currently on the market. Over the past five years, the growth rate of research in breast cancer and myeloma has been particularly noteworthy.
In non-cancerous diseases (Figure 1B), research on autoimmune diseases, inflammation, and infections has taken the lead. In the context of neurodegenerative diseases like Alzheimer’s, Parkinson’s, and Huntington’s, there’s a rising interest in ADC research, indicating a growing curiosity in developing ADCs targeting the brain.
(Data source: CAS website)
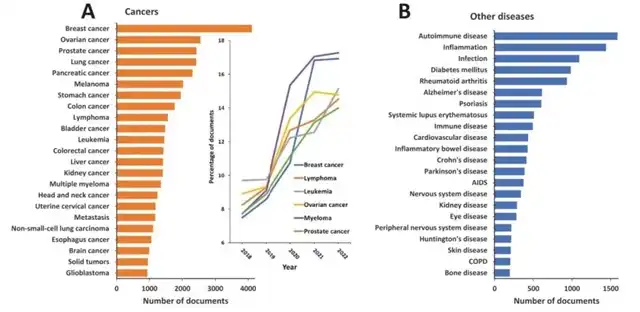
Figure 1 Diseases discussed in ADC-related publications
1. ADC Clinical Development Speeds Up, Indications Expand
1.1 Preclinical Development
Currently, more than 50 organizations globally are researching over 160 preclinical ADC candidates. Some companies have disclosed the target antigens for their ADCs, including HER3, Nectin-4, Folate receptor α, B7H3, CD99, and IGF1R, with many of these candidates in leading positions. About 50% of disclosed preclinical indications are for broad solid tumors and hematologic malignancies, making solid tumor research projects highly favored in preclinical development.
In addition to tumors, ADCs also show promise in autoimmune diseases, inflammation, immune disorders, and challenging conditions like bacterial infections and atherosclerosis. Corresponding payloads include glucocorticoid receptor modulators, kinase inhibitors, antibiotics, and siRNA. Notably, anti-infective ADCs (antibody-antibiotic conjugates) and immune modulator ADCs present particularly promising prospects.
The recent FDA approval of antibody therapy for Alzheimer’s has stimulated the development of ADCs in the field of neurological diseases. Researchers are exploring endogenous macromolecular transport pathways, such as receptor-mediated transcytosis (RMT) and carrier-mediated transport, to facilitate ADC passage through the blood-brain barrier (BBB). Other ADC delivery strategies include ultrasound, microbubbles, and direct injection into the brain.
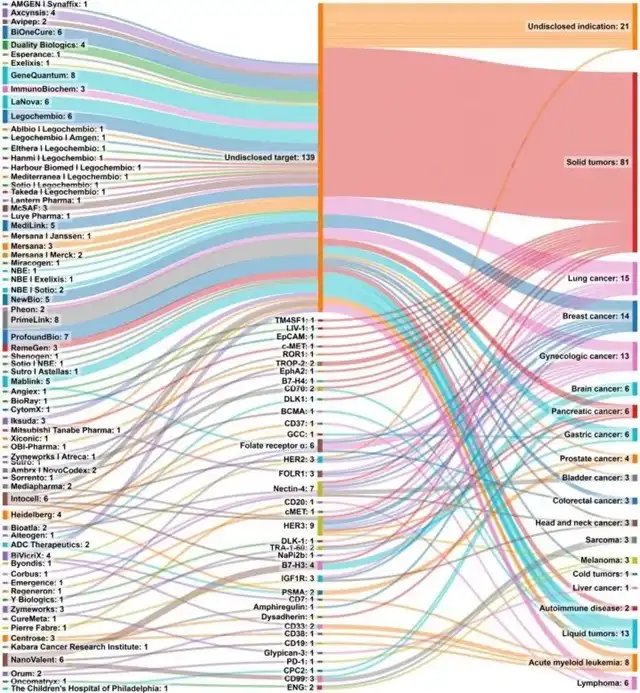
Figure 2 Number of ADC drug candidates (left), target antigen (middle), and disease indication (right) in organizations conducting preclinical ADC research
1.2 Clinical Development
Examining representative ADC clinical trials provides comprehensive insights into past, present, and future clinical development status. These trials indicate a gradual growth of ADCs in clinical development, with an accelerating trend over the past two years. Specifically, almost all ADC clinical trial indications are cancer-related, with approximately 87% in early-stage clinical trials, and the solid tumor pipeline being more active.
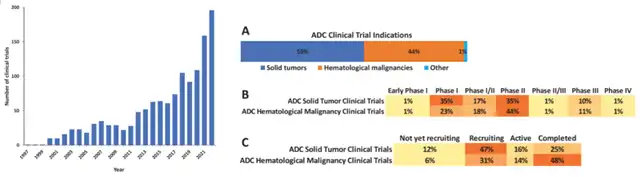
Figure 3 Number of ADC clinical trials per year (left) and ADC clinical indications (A); percentage of each clinical stage (B); percentage of clinical status (C) (right)
Breast cancer, gastric cancer, bladder cancer, lung cancer, leukemia, and lymphoma exhibit more mature positions in the clinical pipeline, with the highest proportion in late-stage trials.
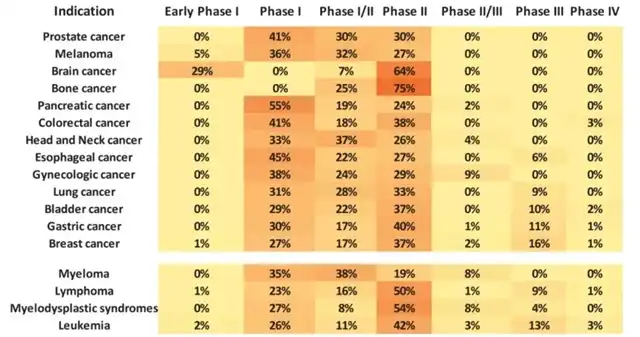
Figure 4 Percentage of each phase of clinical trials of ADCs used to treat specific tumors
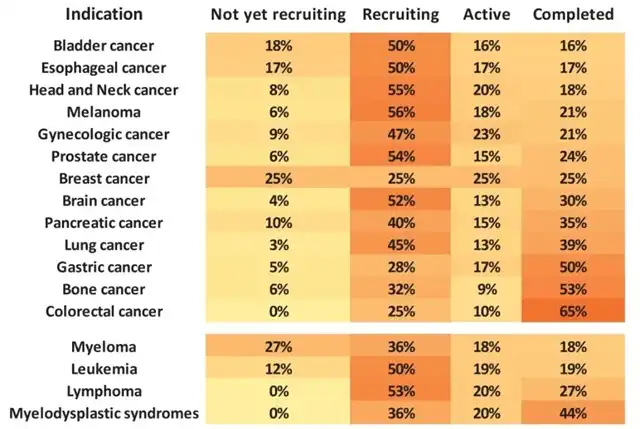
Figure 5 Percentage of various states in clinical trials of ADCs used to treat specific tumors
1.3 Approved ADCs
Currently, there are 15 ADCs globally approved by regulatory authorities. In addition to individual approvals, combination therapies involving ADCs, such as the FDA’s accelerated approval of enfortumab vedotin-ejfv (Padcev, Astellas Pharma) and pembrolizumab (Keytruda, Merck) on April 3, 2023, for treating locally advanced or metastatic urothelial carcinoma patients ineligible for cisplatin chemotherapy, showcase the evolving landscape. Furthermore, late-stage clinical trials are ongoing for Dato-DXd in combination with Keytruda plus platinum-based chemotherapy and Trodelvy with Keytruda, both targeting non-small cell lung cancer.
2. Significant Advances in ADC Development and Utilization
— Combination Therapy
ADCs face several resistance mechanisms, including:
- Downregulation/alteration of antigen expression.
- Reduced internalization of ADC bound to antigens.
- Inefficient/incomplete/improper degradation of ADC within lysosomes.
- Poor lysosomal release of ADC.
- Overexpression of efflux pumps transporting payload extracellularly.
These resistance mechanisms diminish the effectiveness of ADCs in cancer treatment. Combining therapies helps circumvent resistance and associated toxicities, enhancing overall therapeutic efficacy.
2.1 ADC + Conventional Chemotherapy
Combining targeted therapies like ADCs with conventional non-targeted chemotherapy methods can overcome chemotherapy’s limitations and improve survival rates. Notable combinations include gemcitabine (interfering with DNA synthesis) with SGN-35 (CD30 ADC), which is currently in clinical trials across various cancers. Though the exact effectiveness mechanism is unclear, hypotheses suggest that SGN-35 and gemcitabine target Reed Sternberg cells and stromal cells, respectively, with a synergistic effect.
Combining gemcitabine with T-DM1 (HER2 ADC) enhances T-DM1’s anti-tumor effects in pancreatic ductal adenocarcinoma. The level of surface antigen expression is crucial for ADC success, and gemcitabine increases HER2 expression by up to 2.5 times, thereby enhancing T-DM1 efficacy.
Combining gemcitabine with ADCT-301 (CD25 ADC) or Oba01 ADC (DR5 ADC) shows observed synergistic effects. Other explored combinations include alkylating agents like cisplatin, carboplatin, cyclophosphamide, doxorubicin, and ADCs targeting cell surface glycoproteins (LYPD3, CD205, LRG1).
Additionally, combinations of ADCs with antibody therapies are being explored. Anti-angiogenic drugs can promote ADC penetration and exposure to tumor cells. Combination therapies like anetumab ravtansine (MSLN ADC) or mirvetuximab soravansine (FRα ADC) with bevacizumab show enhanced efficacy in preclinical models of ovarian cancer. Recent studies combining mirvetuximab soravtansine and bevacizumab in ovarian cancer patients have shown promising results in key AURELIA trials. Anetumab ravtansine in combination with bevacizumab is also under investigation for ovarian cancer. Trials such as KAITLIN, KRISTINE, and MARIANNE are designed around the synergistic action of T-DM1 and pertuzumab.
2.2 ADC + Immune Checkpoint Inhibitors
Immune checkpoint molecules regulate the degree, type, and duration of immune responses. These molecules play a crucial role in preventing excessive immune activity but often act as accomplices for tumor cells, aiding them in escaping immune surveillance. Well-known inhibitory immune checkpoint molecules include PD-1, CTLA4, BTLA, and LAG3, while stimulatory ones include CD134 and 4-1BB.
As immune checkpoint molecules are expressed on the cell surface, they regulate T-cell activity, making this characteristic advantageous for ADCs. Immune checkpoint modulators can evoke immune responses against cancer, activating T cells to kill cancer cells. Immune checkpoint inhibitors (ICIs) have received FDA approval and are particularly useful in treating solid tumors, even achieving long-term remission. However, ICI therapy is ineffective against triple-negative breast cancer post-chemotherapy due to TP53 mutations altering immune checkpoint molecule expression levels. Similar to other treatment modalities, resistance reduces the efficacy of ICIs, leading to their frequent use in combinations or with conventional chemotherapy to enhance therapeutic effects.
Recently, there is a growing trend of combining ADCs with immunotherapeutic drugs, where ADCs trigger an immune response against cancer cells, suggesting a synergistic effect with immune checkpoint modulators. The most common combinations involve pembrolizumab and nivolumab (PD-1 inhibitors), followed by atezolizumab, durvalumab, and tremelimumab. These combinations are currently in different stages of clinical trials.
Most ADCs used in combination with immunotherapy target HER2, with a few targeting LIV-1, nectin-4, TROP2, B7-H3, among other markers. Recently, ADCs have been developed to simultaneously target immune checkpoint molecules like PD-L1 or B7-H3, preventing immune suppression by directly binding to these molecules and carrying cytotoxic payloads. These dual-function ADCs, connected to anti-PD-L1 antibodies via cleavable disulfide bonds, are termed immune modulating ADCs (IM-ADCs). The payload released by IM-ADCs induces CD8+ and CD4+ T cell cytotoxic lymphocyte infiltration, contributing to their anti-tumor effects. Additionally, the payload increases PD-L1 expression, particularly in tumor cells, enhancing the effectiveness of subsequent treatment using the same ADC.
Moreover, tumor-associated macrophages (TAMs) are a crucial component of the tumor microenvironment (TME). The presence of TAMs is associated with the anti-tumor efficacy of anti-CD30 antibodies (SGN-30, SGN-40). TAMs can also enhance the uptake of non-targeted ADCs and the release of payloads, strengthening bystander effects and effectively killing tumors with low antigen expression or variable antigens.
2.3 Sequential/Intermittent Treatment
Sequential or intermittent therapy involves arranging multiple cancer therapies in a specific order with interspersed pauses, where the drug sequence is critical. This approach maximizes anti-tumor effects while minimizing toxicity. Increasing evidence suggests that in certain situations, such as immunotherapy, sequential treatment may be more effective than combination therapy.
ADC sequential therapy has shown promise in breast cancer, renal cancer, and lung cancer. For instance, compared to combination therapy, cBR96-maytansinoid ADC is more effective when administered before paclitaxel, increasing tumor cell sensitivity to paclitaxel.
3. Outlook: Limitless Potential for ADCs
ADCs rightfully claim their position as a hot topic in current cancer treatment. ADCs in preclinical and clinical development have been predominantly focused on treating tumors. Numerous breakthroughs in ADC drugs for cancer can be observed.
Beyond cancer, ADCs have the potential to be applicable in any disease area.
However, the technology for using ADCs in non-cancerous diseases is not yet mature, and the development process is challenging. Challenges include identifying specific surface markers expressed on target cell types and payloads. To date, there are relatively few ADCs designed for non-cancer indications, and none have successfully passed through clinical trials to enter the market.
With advancements in ADC platforms and technologies, ADCs will not be confined to the cancer treatment domain. More ADCs for non-tumor indications are expected to be developed.
More Than Just Cancer: ADCs Find Applications Across Various Disease Areas
Reference:
Sasso JM, Tenchov R, Bird R, Iyer KA, Ralhan K, Rodriguez Y, Zhou QA. The Evolving Landscape of Antibody-Drug Conjugates: In-Depth Analysis of Recent Research Progress. Bioconjug Chem. 2023 Oct 11. doi: 10.1021/ACS.bioconjchem.3c00374. Epub ahead of print. PMID: 37821099.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



