Milestone: First Twice-Yearly HIV Preventive Therapy Achieves Zero Infections
- Major Breakthrough in Infertility Research: This Stem Cell Could Be Key to IVF and Other Fertility Treatments
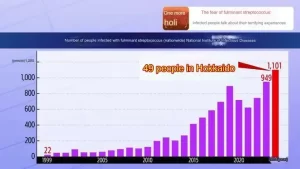
- Flesh-Eating Bacteria Infection Over 1000 Cases in Japan!
- CDC Recommends Updated COVID-19 Vaccines for 2024-2025 Season
- Will China and India produce cheaper “Miracle Weight Loss Drug”Semaglutide soon?
- Keto Diet Accelerates Aging and Promotes Cancer Metastasis
- The Critical Role of Immune Cell Triumvirates in Enhancing CD8+ T Cell Function
Milestone: First Twice-Yearly HIV Preventive Therapy Achieves Zero Infections
- Chinese-made Drug Enters Australia: Causing at Least 20 Deaths!
- How serious is Japan’s “flesh-eating bacteria” problem?
- Taiwan 6th wave of COVID outbreak: 623 confirmed cases in one week and 38 deaths
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Milestone: First Twice-Yearly HIV Preventive Therapy Achieves Zero Infections
On June 20, Gilead Sciences announced the top-line results of a pivotal Phase 3 clinical trial, PURPOSE 1. The interim analysis revealed that the HIV-1 capsid inhibitor lenacapavir, administered biannually, demonstrated 100% efficacy in preventing HIV infections among cisgender women.

Image Source: Gilead Official Website
This is the first Phase 3 clinical trial in HIV prevention to show zero infections. Dr. Merdad Parsey, Gilead Sciences’ Chief Medical Officer, remarked that the results indicate lenacapavir’s potential to be a groundbreaking preventive measure against HIV when administered twice a year. He expressed optimism about further findings from ongoing PURPOSE trials, which aim to contribute to the global fight against HIV.
First HIV Prevention Drug Achieves Zero Infections in Late-Stage Trial
Gilead Sciences is renowned for developing antiviral drugs and has already received approval in China for two HIV pre-exposure prophylaxis (PrEP) medications: emtricitabine/tenofovir disoproxil fumarate (Truvada) in 2020 and emtricitabine/tenofovir alafenamide (Descovy) earlier this year.
The PURPOSE 1 trial is a double-blind, randomized Phase 3 study assessing the safety and efficacy of subcutaneous lenacapavir (administered twice yearly) versus daily oral Descovy (emtricitabine 200mg/tenofovir alafenamide 25mg). The trial involved over 5,300 cisgender women and adolescent girls aged 16-25 from 25 locations in South Africa and three in Uganda, who were randomized into lenacapavir, Descovy, and Truvada groups in a 2:2:1 ratio. Remarkably, no HIV infections occurred among the 2,134 women in the lenacapavir group (incidence rate: 0.00 per 100 person-years).
Gilead reported that PURPOSE 1 met its primary efficacy endpoint, demonstrating the superiority of lenacapavir administered twice yearly compared to daily Truvada and the background HIV infection rate. Lenacapavir was well-tolerated with no significant new safety concerns.
Detailed data from the PURPOSE 1 trial will be presented at future conferences. Gilead is also conducting other key trials for lenacapavir, with results from the PURPOSE 2 study expected by late 2024 or early 2025. PURPOSE 2 evaluates the efficacy of biannual lenacapavir in men who have sex with men (MSM) and other groups across Argentina, Brazil, Mexico, Peru, South Africa, Thailand, and the United States. Positive results from these studies could lead to regulatory approval for lenacapavir as a PrEP option for various high-risk groups and communities.
Dr. Linda-Gail Bekker, Director of the Desmond Tutu HIV Centre at the University of Cape Town and former President of the International AIDS Society, commented that while traditional PrEP medications are highly effective when taken as prescribed, lenacapavir’s biannual administration could address challenges related to the stigma and discrimination associated with oral PrEP and improve adherence and continuity in PrEP usage.
A New Dawn in the Fight Against AIDS
The fight against AIDS has been challenging due to the lack of a cure and difficulties in control. Currently, no curative medications or preventive vaccines exist. Post-exposure prophylaxis (PEP) within 72 hours of high-risk exposure remains the last line of defense against HIV infection.
Once diagnosed, individuals must undergo lifelong antiretroviral therapy (ART), which often leads to issues such as drug resistance and poor patient adherence. According to the United Nations, over half of the global HIV population receives ART.
Experts emphasized that early detection and treatment are crucial for reducing viral loads, promoting immune reconstruction, and lowering the incidence of AIDS-related opportunistic infections or tumors. Early treatment also helps reduce HIV transmission and alleviates the economic burden on individuals and society.
Due to the challenges in developing an effective AIDS vaccine, research has shifted towards long-acting formulations. experts also stated that inconsistent adherence to ART can lead to insufficient drug levels in the body, reducing viral suppression and increasing the risk of drug resistance. Long-acting formulations can lower the frequency of administration, maintain effective drug levels, and improve adherence.
Gilead’s announcement has garnered significant global attention. If approved by the FDA, lenacapavir would become an important new option for HIV prevention. PrEP is a crucial tool in preventing HIV infection, and lenacapavir’s advantage lies in its biannual administration, which enhances adherence.
Milestone: First Twice-Yearly HIV Preventive Therapy Achieves Zero Infections
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.