New Targeted Therapy for Small Cell Lung Cancer (SCLC) Targeting DLL3
- Dispute Intensifies: Moderna Achieves Partial Victory in mRNA Patent Battle with BioNTech and Pfizer
- Harvard Study Finds Semaglutide May Increase Risk of Blindness
- Hundreds of Sperm Donations by One Man Lead to Social Issues in Australia
- Lactic Acid: The Unexpected Ally of Cancer Cells in DNA Repair and Chemoresistance
- Taking Multivitamins May Increase Mortality Rates?
- Why Everyone Should Learn CPR and AED Use?
New Targeted Therapy for Small Cell Lung Cancer (SCLC) Targeting DLL3
- Chinese-made Drug Enters Australia: Causing at Least 20 Deaths!
- How serious is Japan’s “flesh-eating bacteria” problem?
- Taiwan 6th wave of COVID outbreak: 623 confirmed cases in one week and 38 deaths
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
New Targeted Therapy for Small Cell Lung Cancer (SCLC) Targeting DLL3
Small Cell Lung Cancer (SCLC) is an aggressive, high-grade neuroendocrine carcinoma (NEC), accounting for 13%-15% of cancer diagnoses annually. Patients diagnosed with SCLC have a poor prognosis, with a 5-year survival rate ranging from 27% for limited-stage disease to 3% for metastatic disease. Most patients fail to achieve long-term disease control with existing treatments.
Currently, the first-line standard of care (SOC) for SCLC involves platinum-based chemotherapy, with radiotherapy for patients with limited-stage SCLC (LS-SCLC), followed by prophylactic cranial irradiation for those achieving complete remission, and the use of PD-L1 inhibitors combined with chemotherapy for extensive-stage SCLC (ES-SCLC) patients.
Topotecan was the only approved second-line treatment for SCLC for over 20 years until the accelerated approval of the RNA polymerase inhibitor lurbinectedin in 2020 for relapsed SCLC. The limited improvement in treatment efficacy and the almost inevitable development of resistance and relapse after first-line chemotherapy have driven the continuous exploration for more effective treatments.
Delta-like ligand 3 (DLL3) is a Notch inhibitory ligand and an attractive therapeutic target for SCLC due to its overexpression on SCLC cell surfaces and minimal expression on normal cells. Several DLL3-targeted therapies are in development for treating SCLC and other neuroendocrine carcinomas, including antibody-drug conjugates (ADCs), T-cell engagers (TCEs), and chimeric antigen receptor (CAR) therapies.
DLL3 and Small Cell Lung Cancer
In the search for SCLC treatment targets, the transcription factor achaete-scute homolog 1 (ASCL1) has garnered particular attention for its role as a key regulator of neuroendocrine differentiation and its ability to drive SCLC formation. ASCL1 is upregulated in some SCLCs and drives SCLC disease progression and cell survival by regulating the expression of several oncogenes, including MYCL1, RET, SOX2, NFIB, BCL2, and the DLL3 gene, which encodes an inhibitory ligand that suppresses Notch signaling in SCLC cells.
The Notch pathway is evolutionarily conserved, and Notch signaling in SCLC is associated with various carcinogenic processes, including cell proliferation, neuroendocrine plasticity, differentiation, chemotherapy resistance, and regulation of the immune microenvironment. DLL3, a non-canonical Notch ligand, promotes SCLC cell growth and enhances their migratory and invasive capabilities through its overexpression. DLL3 also establishes a metastatic and treatment-resistant phenotype in NECs by promoting cell proliferation and acquiring resistance to platinum-doublet chemotherapy.
Up to 85% of human SCLC tumors express DLL3 protein on their cell surfaces. Besides SCLC, DLL3 is also widely expressed in other NECs, including those of the lung (certain molecular subtypes of large cell NEC), gastrointestinal tract, pancreas, bladder, prostate, and cervix. High DLL3 expression is associated with poorer outcomes and worse survival in these tumors.
DLL3-Targeted ADCs
Rova-T is a DLL3-targeted ADC consisting of a DLL3-specific humanized monoclonal antibody (SC16) conjugated to a pyrrolobenzodiazepine (PBD) dimer toxin via a cleavable dipeptide linker. The binding of Rova-T to cell surface DLL3 triggers the internalization of the ADC-target complex, and the VA linker of Rova-T is subsequently cleaved by lysosomal cathepsin B, releasing PBD into the cytoplasm. PBD then enters the nucleus, cross-links DNA, and induces tumor cell death via apoptosis.
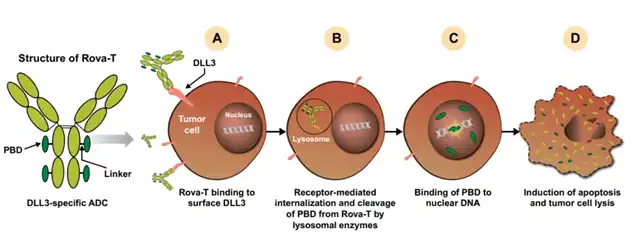
Rova-T has undergone at least 10 clinical trials, including two phase 3 trials. Its first-in-human (FIH) study in second- and third-line treated patients produced an 18% objective response rate (ORR) with a 1-year survival rate of 36%, which was impressive.
In the phase 2 TRINITY study, Rova-T achieved an ORR of 12.4% and a median overall survival (OS) of 5.6 months in 339 DLL3-expressing SCLC patients. The ORR was slightly higher at 14.3% in patients with high DLL3 expression, with a median progression-free survival (PFS) of 3.8 months and a median OS of 5.7 months.
The phase 3 TAHOE (Rova-T versus topotecan as second-line therapy) and MERU (Rova-T as maintenance therapy post first-line treatment) trials were terminated early due to not meeting pre-specified interim PFS and/or OS primary endpoints. In the TAHOE trial, the median OS was 6.3 months for the Rova-T group compared to 8.6 months for the topotecan group. In the MERU trial, the median OS was 8.8 months for the Rova-T group versus 9.9 months for the placebo group; analysis limited to high DLL3 expressors did not improve outcomes. These clinical findings, along with other considerations, led to the discontinuation of Rova-T development.
Safety-wise, Rova-T in phase 1-3 trials for platinum-refractory SCLC observed grade 3 treatment-related adverse events (TRAEs) in 38%-64% of patients and grade 5 TRAEs in 1.7%-7.1% of patients. The most common TRAEs were thrombocytopenia, pleural effusion, photosensitivity reaction, and anemia.
DLL3-Targeted T-Cell Engagers
T-cell engagers (TCEs) have dual specificity, enabling them to bind both the CD3 complex on T-cells and the target antigen on tumor cells. This dual binding brings tumor cells into close proximity with autologous T-cells, triggering immune synapse formation and T-cell activation, ultimately leading to tumor cell apoptosis and amplification of the T-cell response. Multiple DLL3-targeted TCEs are in development, with tarlatamab leading the way, having entered phase 3 clinical trials in 2023.
Tarlatamab
Tarlatamab consists of two single-chain variable fragments (scFvs) and includes a stable, effector-less Fc domain to increase half-life. Six ongoing clinical trials are evaluating tarlatamab, including five for SCLC patients and one phase 1 trial for neuroendocrine prostate cancer (NEPC) patients.
Tarlatamab’s FIH study enrolled 107 patients with progressive or relapsed SCLC after at least one prior treatment, with dose levels ranging from 0.003 mg to 100 mg. A step-up dosing regimen was adopted from the 3 mg cohort to mitigate cytokine release syndrome (CRS) observed in previous cohorts. Responses began to appear at the 0.3 mg dose level, with confirmed responses in 25 patients (ORR: 23.4%), including 2 complete responses (1.9%) and 23 partial responses (21.5%), with 30 patients (28%) achieving stable disease (SD). The disease control rate was 51.4%, with a median PFS of 3.7 months and a median OS of 13.2 months. The median time to response was 1.8 months, and the median duration of response (DOR) was 12.3 months, indicating that most responders showed early responses, with response durations encouraging compared to other SCLC treatments.
Safety-wise, the ongoing DeLLphi-300 FIH trial (NCT03319940) reported that 90.7% (97/107) of patients experienced any grade TRAE, with 30.8% (33/107) experiencing grade ≥3 TRAEs. Four patients (3.7%) discontinued treatment due to TRAEs, with no grade 5 TRAEs observed. CRS, characterized by mild fever and/or hypotension, was the most common TRAE, occurring in 52.3% (56/107) of patients. CRS typically occurred in the first treatment cycle and was manageable with supportive care. Additionally, 53 patients (49.5%) experienced treatment-related neurological adverse events, mostly mild (grade 1), with the most common being taste disturbance (22.4%), headache (10.3%), confusion (5.6%), and dizziness (5.6%).
HPN328
HPN328 is a trispecific T-cell activating construct (TriTAC) comprising three humanized antibody-derived domains: an N-terminal domain binding DLL3 on tumor cells, a middle domain binding human serum albumin (for half-life extension), and a C-terminal domain binding CD3.
Preliminary results from a phase 1 trial of HPN328 have been disclosed. Eighteen SCLC and other NEC patients were treated with doses ranging from 0.015 mg/week to 12.0 mg/week, using step-up dosing for higher doses. Overall, 3 of 11 SCLC patients (27%) achieved partial responses, including one SCLC patient with a 33% SD. CRS occurred in 22% of patients at grade 1-2 levels; no grade 3 or higher CRS events were observed at tested doses, and the maximum tolerated dose has not yet been reached.
Other TCEs
Other DLL3/CD3 TCEs include BI 764532 and QLS31904. BI 764532 is a bispecific DLL3/CD3 antibody with an IgG backbone. QLS31904 is another TCE, comprising a DLL3-specific Fab fragment, a CD3-specific Fab fragment, and an Fc domain.
DLL3-Targeted CAR T-Cell Therapy
AMG119
AMG119 is an autologous DLL3-specific CAR T-cell product currently undergoing a phase 1 trial in patients with recurrent or progressive SCLC after prior systemic treatment. Preliminary findings indicate that all six patients dosed thus far experienced TRAEs, including grade 3 TRAEs in three patients. Notably, no grade 4/5 TRAEs were observed, and no dose-limiting toxicities (DLTs) or serious adverse events (SAEs) related to AMG119 were reported. CRS occurred in 50% of patients at grade 1-2 levels, with no grade 3 or higher CRS events reported. Additionally, neurotoxicity was absent in all six patients.
Future Prospects
The development of DLL3-targeted therapies represents a significant breakthrough in the treatment of SCLC, a disease with historically limited treatment options and poor outcomes.
While Rova-T did not achieve the desired clinical outcomes, other DLL3-targeted therapies like tarlatamab, HPN328, and AMG119 show promise and are undergoing clinical trials. Further research and clinical trials will determine the long-term efficacy and safety of these therapies in improving SCLC patient outcomes.
New Targeted Therapy for Small Cell Lung Cancer (SCLC) Targeting DLL3
Reference:
1.Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J Hematol Oncol. 2023; 16: 66.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.