What are the commonly used HER2 targeted drugs for breast cancer?
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Gut Bacteria Enzymes Offer Hope for ABO Universal Blood Transfusions
- Well-Known Japanese Medicine Exposed for 30 Years of Data Falsification
- Oregon Reverses Course: From Decriminalization to Recriminalization of Drug Possession
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
What are the commonly used HER2 targeted drugs for breast cancer?
What are the commonly used HER2 targeted drugs for breast cancer? Trastuzumab, lapatinib…
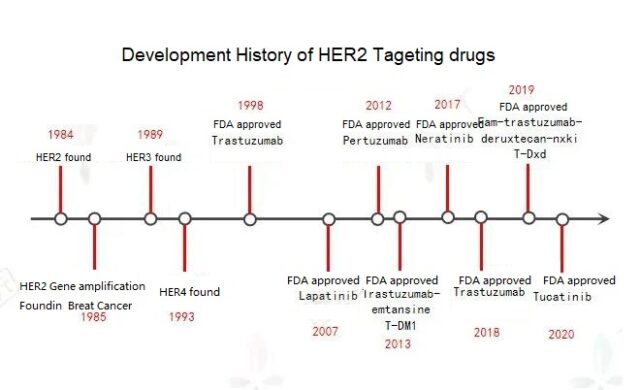
Around 1984, scientists discovered a gene similar to human epidermal growth factor receptor HER (EGFR) and named it HER2. The human epidermal growth factor receptor (HER; EGFR) family has 4 members-HER1 (EGFR), HER2, HER3 and HER4.
Breast cancer is the malignant tumor with the highest incidence in women today, and the amplification of the HER2 gene is an early event of breast cancer carcinogenesis. Among breast cancer patients, about 20%-30% of patients are HER2 positive.
HER2 positive means that the gene plays a role, inhibits cell apoptosis, promotes proliferation, and greatly increases the invasiveness of cancer cells. The same also means that HER2-positive breast cancer is more malignant and more prone to disease progression. It is easier to relapse and metastasize.
Therefore, HER2 has become one of the popular targets. Since the advent of HER2 targeted preparations, the treatment pattern of HER2-positive breast cancer patients has been greatly changed.
At present, anti-HER2 drugs are mainly divided into three categories: monoclonal antibodies, small molecule tyrosine kinase inhibitors, and antibody-conjugated drugs (ADC).
- Monoclonal antibodies bind to HER2 receptors to prevent the formation of HER2 dimers or the immune response to inhibit the decomposition of HER2, ultimately destroying downstream signaling pathways.
- Small molecule tyrosine kinase inhibitors bind to HER2 intracellular tyrosine kinases to inhibit the phosphorylation of tyrosine kinases, thereby inhibiting the activity of downstream pathways.
- The ADC mechanism combines with the extracellular structure of HER2 to form a complex, which induces endocytosis and allows the drug to work.
01. Trastuzumab
In 1998, the world’s first HER2 preparation, trastuzumab (Herceptin), achieved great success once it was launched on the market. This is currently the most commonly used drug for HER2-positive breast cancer. It is a monoclonal antibody that directly binds to HER2 on the surface of cancer cells to activate lymphocytes to release cytotoxic substances and promote cancer cell apoptosis. More than 70% of early breast cancer patients are cured after using Herceptin; Herceptin combined with standard chemotherapy can significantly improve the disease-free survival rate of patients with metastatic cancer and prolong the survival time of patients.
Recommended dose: 8 mg/kg loading dose/6 mg/kg q3w intravenous infusion.
Indications: HER2-positive metastatic breast cancer. As a single-drug treatment for metastatic breast cancer that has received one or more chemotherapy regimens; combined with paclitaxel or docetaxel for metastatic breast cancer patients who have not received chemotherapy. The effective rate of the drug alone is not high, only about 10-20%. It is clinically mainly used in combination with chemotherapy or other anti-HER2 targeted drugs, and the effective rate can reach more than 60%.
Adverse reactions: The most common adverse reactions (> 25%) are fatigue, nausea, musculoskeletal pain, thrombocytopenia, headache, elevated transaminase and constipation. The combination of trastuzumab and cardiotoxic anthracycline chemotherapeutics is generally not recommended.
02. Lapatinib
Although Herceptin combined with chemotherapy is effective in the treatment of most HER2-positive breast cancers, there are still many patients who eventually have disease recurrence or progression, and drug resistance is one of the main reasons. In order to overcome drug resistance, some small molecule inhibitors with a different mechanism of action from Herceptin came into being. Lapatinib, which was approved by the FDA in 2007, is an oral small molecule tyrosine kinase inhibitor targeting both HER1 and HER2 targets.
Recommended dose: The recommended dose is 1250 mg, once a day, on days 1-21, combined with capecitabine 2000 mg/d; on days 1-14, it is taken twice, repeated every three weeks.
Indications: Lapatinib and capecitabine are used in combination. The NCCN guidelines recommend it for the treatment of patients with advanced or metastatic breast cancer who are HER2-positive and have previously received treatments including anthracyclines, paclitaxel, and trastuzumab. It must be used by patients with recurrence and metastasis who have progressed after receiving trastuzumab treatment. The effective rate of advanced first-line single-agent therapy is about 12-24%, general clinical guidelines recommend second-line medication, and the effective rate of combined treatment with capecitabine is about 26-33%, and the clinical benefit rate can reach up to 71.3%, which is the most commonly used Of lapatinib combined with chemotherapy.
Adverse reactions: Mainly gastrointestinal reactions and hand-foot syndrome, including nausea, diarrhea, stomatitis and indigestion, etc., dry skin, rash, back pain, difficulty breathing and insomnia. When combined with capecitabine, the incidence of diarrhea, hand-foot syndrome, and rash may be as high as 55%, 46%, and 43%, respectively.
03. Pertuzumab
Pertuzumab, which was launched in the United States in June 2012, is similar to trastuzumab. Both act on different target areas of HER2, inhibiting the proliferation of HER2 overexpressing tumor cells, thereby slowing the growth of tumors. The latest research shows that “toto” dual-target combined chemotherapy, compared with trastuzumab combined with chemotherapy, can prolong the median overall survival of advanced patients by 15.7 months.
Recommended dose: The initial dose is 840 mg, and the infusion is completed within 60 minutes. Thereafter, 420 mg was given by intravenous infusion every 3 weeks for 30-60 minutes.
Indications: Approved to combine trastuzumab and chemotherapy for adjuvant treatment of HER2-positive early breast cancer patients with a high risk of recurrence; combined with trastuzumab and chemotherapy for HER2-positive, locally advanced, inflammatory Or neoadjuvant therapy for patients with early breast cancer (diameter> 2 cm or lymph node positive); combined with trastuzumab and docetaxel for first-line HER2-positive metastatic breast cancer patients who have not received anti-HER2 therapy or chemotherapy Standard treatment.
The most common adverse reactions (≥ 30%) are: diarrhea, hair loss, nausea, fatigue, neutropenia and vomiting.
04. T-DM1
Hercelium (T-DM1) is China’s first approved antibody-conjugated drug (ADC), which is a combination of trastuzumab (T) and the chemical drug maytansine derivative (DM1) to make the antibody band Using chemotherapeutics to find tumor cells overexpressing Her-2 not only avoids the side effects of systemic chemotherapeutics, but is also more lethal than targeted drugs alone.
Recommended dose: 3.6 mg/kg intravenous infusion every 3 weeks (21 days as a cycle), administration until disease progression or unacceptable toxicity.
Indications: Currently approved for HER2-positive metastatic breast cancer, it is the second-line first choice after trastuzumab treatment fails. If there is early progression after or during adjuvant therapy, T-DM1 is the first-line palliative treatment of choice.
The most common adverse reactions are fatigue, nausea, musculoskeletal pain, thrombocytopenia, headache, elevated transaminase and constipation.
05. Nelatinib/lenatinib
Nelatinib, launched in 2017, is an irreversible small molecule tyrosine kinase inhibitor targeting HER1, HER2, and HER4.
Recommended dose: 240 mg (6 tablets) orally once a day for one year. It is recommended to perform dose interruption and/or dose reduction based on personal safety and tolerability.
Indications: Prolonged adjuvant therapy for adult patients with early HER2-positive breast cancer who have previously received adjuvant trastuzumab; combined with capecitabine for metastatic advanced HER2-positive patients who have received two or more treatment options Breast cancer patients.
Adverse reactions: Diarrhea is the most common side effect, followed by nausea, abdominal pain, fatigue, vomiting, rash, swelling and stomatitis (stomatitis), loss of appetite, muscle cramps, indigestion, etc.
06. Biosimilars
According to the regulations of the European Union, the United States, and China’s drug regulatory agencies, biosimilar drugs refer to therapeutic biological products that are similar in quality, safety, and effectiveness to reference drugs that have been approved for registration. With the expiration of patents of original biopharmaceuticals and the continuous development of biotechnology, the research and development of biosimilar drugs based on the quality, safety and effectiveness of original biopharmaceuticals will help increase the availability and lower prices of biopharmaceuticals, and meet Drug demand of the masses.
In December 2017, Ogivri, the first trastuzumab biosimilar, was approved by the FDA for marketing. In December 2018, the FDA approved Herzuma, another biosimilar to Herceptin. On January 18, 2019, the FDA announced the approval of Samsung Bioepis’ trastuzumab (Herceptin) biosimilar drug Ontruzant.
07. T-Dxd
T-DXd (DS8201) is a HER2 antibody + irinotecan-based chemotherapeutic drug conjugate, and it also belongs to the ADC type drug type (antibody conjugated drug). Compared with TDM-1, T-DXd has a higher drug-to-antibody ratio (DAR, about 8:3-4), and is more effectively delivered to tumor cells expressing HER2.
On December 20, 2019, the FDA approved T-DXd for patients with unresectable or metastatic HER2-positive breast cancer. Previous studies have shown that overall T-DXd has acceptable safety. So far, there are still 5 phase 3 randomized trials on T-DXd in breast cancer patients.
08. Tucatinib
Tucatinib is an oral bioavailable small molecule tyrosine kinase inhibitor (TKI). It is highly selective for HER2 and has no obvious inhibitory effect on EGFR. In addition, tucatinib has been granted orphan drug designation by the FDA for the treatment of breast cancer patients with brain metastases.
Dosage: The recommended starting dose is 300 mg twice a day. If you experience vomiting or accidentally miss a dose after taking the drug, please do not take it again. Instead, take the next dose directly at the normal time.
Indications: On April 17, 2020, the FDA approved tucatinib in combination with trastuzumab and capecitabine for the treatment of patients with locally advanced unresectable or metastatic HER2-positive breast cancer, including brain metastases Patients, these patients have received at least 3 previous anti-HER2 drugs separately or in combination.
The most common side effects are: diarrhea, hand-foot syndrome, nausea, fatigue, and vomiting.
SUM UP:
In addition to the above-mentioned drugs, there are some new HER2 targeted preparations in clinical trials. For example, Vidicuzumab (RC48) is mainly targeted at locally advanced or metastatic breast cancer with low HER2 expression. Phase III clinical trials have been carried out, while clinical development for HER2-positive breast cancer is in phase II.
In the latest edition of NCCN2021V2 guidelines, Margetuximab (Margetuximab-cmkb) combined with chemotherapy is used as a third-line and above treatment plan. As the family of HER2 targeted agents grows stronger, there will be more options for the treatment of patients with advanced HER2-positive breast cancer in the future.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.