FDA approved HER2-positive gastric cancer first-line immunotherapy!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA approved HER2-positive gastric cancer first-line immunotherapy! Merck’s Keytruda + trastuzumab + chemotherapy program was approved by the U.S. FDA!
FDA approved HER2-positive gastric cancer first-line immunotherapy! Merck & Co. recently announced that the U.S. Food and Drug Administration (FDA) has approved the anti-PD-1 therapy Keytruda (generic name: pembrolizumab, pembrolizumab), combined with trastuzumab (Trastuzumab) and chemotherapy containing fluoropyrimidine and platinum drugs, as the first-line treatment for patients with locally advanced unresectable or metastatic HER2-positive gastric cancer or gastroesophageal junction (GEJ) adenocarcinoma.
It is worth mentioning that Keytruda is the first anti-PD-1 therapy approved for first-line treatment of gastric cancer or GEJ adenocarcinoma in combination with trastuzumab and chemotherapy. This indication is reviewed under the FDA’s Real-Time Oncology Review (RTOR) pilot project, and the tumor response rate and response durability data are given accelerated approval. Continued approval for this indication will depend on the verification and description of clinical benefits in confirmatory clinical trials.
Dr. Roy Baynes, Chief Medical Officer, Senior Vice President, and Head of Global Clinical Development, Merck Research Laboratories, said: “Today’s approval marks an important milestone because this is the first time that anti-PD-1 therapy has been approved in combination with anti-HER2 therapy and chemotherapy. The first-line treatment for these patients. Since the beginning of Keytruda development, we have been constantly exploring new combinations to help more cancer patients. We are very happy to bring a new kind to patients with HER2-positive gastric cancer and GEJ adenocarcinoma. The first-line joint program has a significant improvement in ORR compared to the standard program.”
The latest approval of Keytruda is based on data from the ongoing Phase 3 KEYNOTE-811 trial (NCT03615326). The trial was conducted in 692 patients with HER2-positive advanced gastric cancer or GEJ adenocarcinoma who have not received systemic therapy for metastatic disease. It evaluated Keytruda in combination with trastuzumab and chemotherapy (5-fluorouracil + cisplatin, or capecita Bin + oxaliplatin) for the efficacy and safety of first-line treatment.
The results showed that compared with the trastuzumab + chemotherapy group, the Keytruda + trastuzumab + chemotherapy group showed a statistically significantly higher objective response rate (ORR: 74% vs 52%; p<0.0001). In the Keytruda regimen group, the complete response rate (CR) was 11%, and the partial response rate (PR) was 63%. In the trastuzumab + chemotherapy group, CR was 3.1% and PR was 49%.
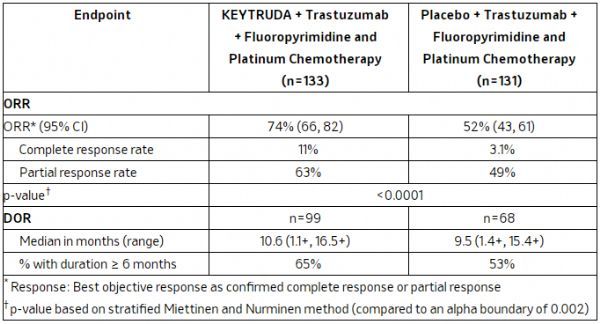
KEYNOTE-811 clinical data
In terms of immunotherapy for gastric cancer, in mid-April this year, Bristol-Myers Squibb’s (BMS) anti-PD-1 therapy Opdivo (Odivo, generic name: nivolumab, nivolumab) was approved by the US FDA, combined with fluoropyrimidine and platinum Class-like combination chemotherapy, first-line treatment of advanced or metastatic gastric cancer (GC), gastroesophageal junction (GEJ) cancer, esophageal adenocarcinoma (EAC) adult patients, regardless of PD-L1 expression status.
Opdivo is the first and only immunotherapy that combines chemotherapy and chemotherapy alone in the first-line treatment of gastric cancer, GEJ cancer, and EAC to significantly improve overall survival (OS), and will become a new standard of care for such patients. The results from the key phase 3 clinical study (CheckMate-649) showed that compared with the chemotherapy group, the Opdivo+ chemotherapy group had statistically significant and clinically significant improvements in overall survival (OS) and progression-free survival (PFS).
The specific data are: Among PD-L1 positive patients with CPS ≥ 5, the median OS of the Opdivo+ chemotherapy group was 14.4 months (95% CI: 13.1-16.2), while the median OS of the chemotherapy group was 11.1 months (95 %CI: 10.0-12.1), the data has a statistically significant difference (HR=0.71; 98.4%CI: 0.59-0.86; p<0.0001). The median PFS of the Opdivo+ chemotherapy group was 7.7 months (95%CI: 7.0-9.2), while that of the chemotherapy group was 6.0 months (95%CI: 5.6-6.9). The data also had statistically significant differences (HR: 0.68; 98%CI: 0.56-0.81; p<0.0001). In this trial, the safety of the Opdivo+chemotherapy combination reflects the known safety of Opdivo and chemotherapy, and no new safety signals have been observed.
Opdivo combined with chemotherapy has also observed statistically significant OS benefits in the PD-L1 positive patient population with CPS≥1 and all randomized patient populations. In all randomized patient groups, the median OS of patients receiving Opdivo+ chemotherapy was 13.8 months (95% CI: 12.6-14.6), and the median OS of patients receiving chemotherapy only was 11.6 months (95% CI: 10.9-12.5) , The data is statistically significant (HR: 0.80; 99.3%CI: 0.68-0.94; p=0.0002).
Among PD-L1 positive patients with CPS ≥ 1, the median OS of patients receiving Opdivo+ chemotherapy was 14.0 months (95% CI: 12.6-15.0), and the median OS of patients receiving chemotherapy only was 11.3 months (95% CI :10.6-12.3), the data is also statistically significant (HR: 0.77; 99.3%CI: 0.64-0.92; p=0.0001).
Stomach cancer is the fifth most common cancer in the world and the third leading cause of cancer deaths. In 2018, there were more than 1 million new cases of gastric cancer worldwide and approximately 783,000 deaths. The definition of gastric cancer is broad. A variety of cancers, including gastroesophageal junction (GEJ) cancer formed at the junction of the stomach and esophagus, can be classified as gastric cancer. Compared with gastric cancer, the prevalence of gastroesophageal junction cancer is low, but it continues to increase.
Keytruda belongs to anti-PD-(L)1 tumor immunotherapy. This type of therapy helps detect and fight tumor cells by improving the ability of the human immune system. Keytruda is an anti-PD-1 therapy that blocks the interaction between PD-1 and its ligands PD-L1 and PD-L2, thereby activating T lymphocytes that may affect tumor cells and healthy cells. At present, Keytruda has become the basic therapy for many types of cancer.
Globally, more than 10 anti-PD-(L)1 therapies have been approved for marketing. Keytruda is the leader among them. In 2020, global sales will reach 14.38 billion U.S. dollars, an increase of 30% over the previous year.
Merck has the industry’s largest immuno-oncology clinical development project. There are currently more than 1,400 clinical trials investigating the role of Keytruda in multiple types of tumors and treatment settings. The Keytruda clinical project aims to understand the role of the drug in cancer and the factors that may predict patients’ benefit from Keytruda treatment, including the exploration of several different biomarkers.
(source:internet, reference only)
Disclaimer of medicaltrend.org



