FDA approves first-line immunotherapy for gastric cancer again
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA approves first-line immunotherapy for gastric cancer again
FDA approves first-line immunotherapy for gastric cancer again for HER2+ gastric or gastroesophageal junction adenocarcinoma!
Stomach or gastroesophageal junction cancer is the fifth most common cancer in the world and the fourth leading cause of cancer death. Chemotherapy can prolong the survival period of stomach or gastroesophageal junction cancer, but their prognosis is still very poor.
For patients with HER2-positive (HER2+) metastatic gastric or gastroesophageal junction cancer, the first-line treatment is recommended to add trastuzumab (anti-HER2) to platinum + fluorouracil chemotherapy drugs. 1,2,3
With the rise of immunotherapy, it is expected to change the clinical practice of gastric cancer. Can immune checkpoint inhibitors + anti-HER2 therapy become a new standard for HER2+ metastatic gastric or gastroesophageal junction cancer? Can it bring new hope to patients with advanced HER2+ gastric or gastroesophageal junction cancer?
On May 5, 2021, the FDA accelerated the approval of Pembrolizumab (Keytruda, K for short) combined with trastuzumab and platinum + fluorouracil chemotherapeutics for HER2 positive (HER2+) locally advanced unresectable or metastatic disease First-line treatment for patients with adenocarcinoma of the stomach or gastroesophageal junction. At the same time, this is also the first PD-1 therapy combined with anti-HER2 therapy and chemotherapy approved by the FDA for first-line immunotherapy for patients with HER2-positive gastric or gastroesophageal junction adenocarcinoma.
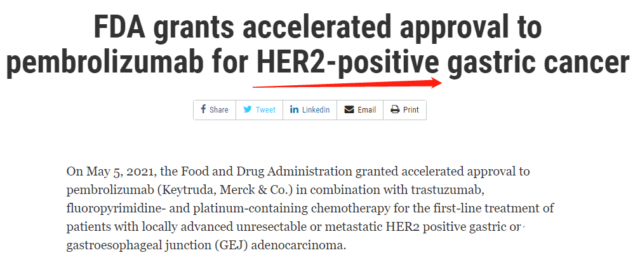
This accelerated approval is based on the interim analysis data of a phase III multicenter, randomized, double-blind, placebo-controlled trial KEYNOTE-811 (NCT03615326), which aims to recruit 692 patients who have not previously received systemic treatment and have HER2 Patients with positive metastatic gastric or gastroesophageal junction adenocarcinoma (cohort 1 below), through PD-L1 expression (CPS≥1 is positive or CPS<1 is negative), chemotherapy regimen (fluorouracil + cisplatin (FP regimen) Or capecitabine + oxaliplatin (CAPOX program)), geographical area (Europe/Israel/North America/Australia, Asia or other parts of the world) randomize (1:1) groups of patients and receive 200mg Pascalin every three weeks Bolivizumab or placebo, combined with trastuzumab and FP regimen or CAPOX regimen, the main research endpoints include progression-free survival (PFS) and overall survival (OS).
*Cohort 2 is a specific cohort of 40 Japanese patients, combined with SOX chemotherapy
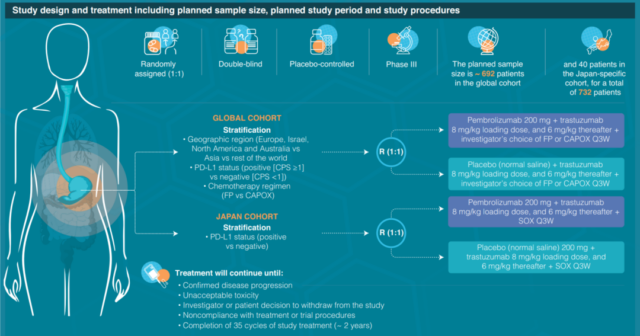
In the interim efficacy analysis, 264 randomized (1:1) patients were evaluated for overall response rate (ORR) and duration of response (DOR). The ORR of the pembrolizumab group was 74%, which was significantly superior In 52% of the placebo group (trastuzumab + chemotherapy), the complete remission rate was 11% vs 3.1%, which was statistically significant. In addition, the median DOR of the pembrolizumab group was 10.6 months, while that of the placebo group (trastuzumab + chemotherapy) was 9.5 months, and the response was also very long-lasting. The safety of pembrolizumab observed in the study is consistent with previous reports.
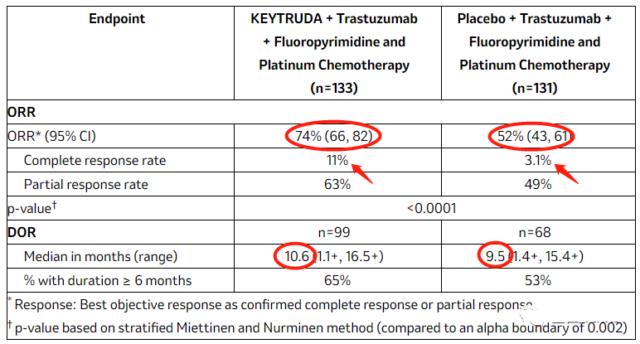
01. Need to detect PD-L1 expression?
No, the key eligibility criteria do not need to look at the PD-L1 status.
The KEYNOTE-811 phase III clinical trial actually detects the expression of PD-L1, using Agilent’s Dako PD-L1 IHC 22C3 pharmDx test to evaluate PD-L1 CPS (comprehensive positive score), where CPS ≥ 1 is positive Or CPS<1 is negative, PD-L1 is a pre-examination marker, as a subgroup analysis.
In recent years, it has been observed that the immune system contributes to the therapeutic effect of HER2 targeting antibodies to a large extent, and trastuzumab treatment may increase the expression of tumor PD-L1, so more and more people are The use of tocilizumab combined with immune checkpoint inhibitors has generated interest. In the currently ongoing phase Ib/II clinical trial of PANTHERA (NCT02901301), the dosing schedule and patient population are similar to those of the KEYNOTE-811 trial. The latest results show: regardless of PD-L1 status, pembrolizumab + trastuzumab Anti-+capecitabine+cisplatin is effective for HER2-positive advanced gastric cancer. The ORR of the first-line treatment is 76.7%, and the DCR is 97.7%. 6 (A similar conclusion was reached in the early trial NCT02954536: regardless of the status of PD-L1, the efficacy is similar 7)
02. Need to detect HER2 status?
It must be tested. The KEYNOTE-811 phase III clinical trial must be confirmed by the central laboratory to be positive for HER2 expression in tumor tissues (IHC 3+ or 2+ combined with ISH/FISH amplification) to be eligible for clinical randomization.
HER2-positive gastric cancer is a unique disease subtype that requires a diagnosis and treatment strategy different from HER2-negative gastric cancer. Patients with HER2-positive advanced gastric cancer can benefit from anti-HER2 therapy or combined immunotherapy. Globally, the positive rate of HER2 overexpression in gastric cancer is 7.3%~20.2%, and the positive rate of HER2 in Chinese gastric cancer patients is 12%~13%.
In addition, the 2020 CSCO Guidelines for Gastric Cancer also pointed out that the HER2 gene somatic copy number results based on ctDNA targeted sequencing in the blood are highly consistent with FISH data. For patients who cannot obtain biopsy tissue, liquid biopsy HER2 amplification is a possible and effective Supplementary means. CtDNA-based HER2 amplification can also be used to monitor the response to trastuzumab treatment in patients with gastric cancer. 9
This approval is an important milestone for patients with HER2-positive gastric or gastroesophageal junction adenocarcinoma, because it is the first time that anti-PD-1 therapy is combined with anti-HER2 therapy and chemotherapy as the first-line treatment for such patients , ORR reached 74% (vs 52%). The Phase III clinical trial of KEYNOTE-811 is still in progress, and we continue to look forward to the results of PFS and OS, the main research endpoints.
(source:internet, reference only)
Disclaimer of medicaltrend.org



