Factors Influencing CAR-T Cell Persistence and Countermeasures
- Keto Diet Accelerates Aging and Promotes Cancer Metastasis
- The Critical Role of Immune Cell Triumvirates in Enhancing CD8+ T Cell Function
- Chinese-made Drug Enters Australia: Causing at Least 20 Deaths!
- UV Rays Threaten Golfers’ Health: Sun Protection Strategies You Need to Know
- RSV: The Hidden Killer of Infants and ToddlersNews draft
- Will Lorlatinib Become A Game-Changer in Treatment for ALK-Positive Lung Cancer?
Factors Influencing CAR-T Cell Persistence and Countermeasures
- Chinese-made Drug Enters Australia: Causing at Least 20 Deaths!
- How serious is Japan’s “flesh-eating bacteria” problem?
- Taiwan 6th wave of COVID outbreak: 623 confirmed cases in one week and 38 deaths
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Factors Influencing CAR-T Cell Persistence and Countermeasures
Over the past decade, significant progress has been made in enhancing the efficacy of CAR-T cell therapy. However, its clinical benefits remain limited, particularly in solid tumors.
Even in hematologic malignancies, patients responding to CAR-T therapy still face relapse risks due to factors like poor T cell expansion and lack of long-term persistence after adoptive transfer.
This issue is more pronounced in solid tumors, where the tumor microenvironment (TME) negatively impacts T cell survival, infiltration, and activity. One hallmark of cancer is the presence of suppressive determinants in the TME that impair T cell function, leading to T cell exhaustion.
Limited persistence remains a major obstacle in the development of CAR-T therapy, starting from the cell manufacturing steps. Factors such as CAR design, in vitro manipulation, and culture conditions play crucial roles. Engineering CAR-T cells to improve their survival and reverse or prevent the exhausted phenotype might be a logical therapeutic approach.
Modern synthetic biology and genome editing technologies offer opportunities, and some studies have already tested methods to limit and create anti-exhaustion T cells in key clinical trials. These methods may address CAR-T cell persistence issues, offering hope for patients.
Factors Influencing CAR-T Cell Persistence
CAR Design
The type of extracellular CAR domain, the spacer connecting the single-chain antibody to the transmembrane domain, and the costimulatory domains constituting the CAR significantly affect T cell function and persistence.
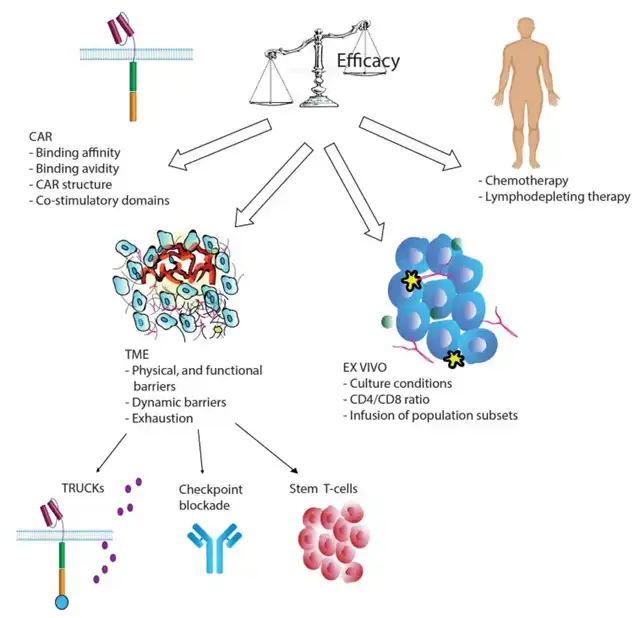
Most CARs used in clinical trials originate from mice, potentially leading to immune rejection mediated by humoral and CD8+ T cell responses. Reinfusing CAR-T cells containing mouse components into patients with CD19+ relapse after initial treatment is largely ineffective. Furthermore, clusters of mouse CAR receptors on the cell surface can generate tonic signals, causing T cell exhaustion. In 2016, humanized CAR-T cells with humanized scFv entered clinical trials, showing similar cytotoxic activity to mouse CAR but enhanced persistence due to lower immunogenicity.
CAR affinity influences tonic signaling; overly strong affinity to target cells can lead to T cell exhaustion. One study developed a CAT-CAR (CD19 single-chain antibody) with 40 times lower affinity for CD19 than traditional CARs. In clinical studies on pediatric patients with refractory or relapsed BLL, 11 of 14 patients with increased CAR-T cell expansion showed persistence.
Additionally, costimulatory domains play key roles in CAR-T cell persistence and efficacy, with costimulatory molecules including CD28, ICOS, CD27, 4-1BB, OX40, and CD40L. Several studies found that CAR constructs equipped with 4-1BB domains, rather than CD28, enhanced T cell persistence. Other studies reported that 4-1BB CAR-T cells exhibited an enhanced memory T cell (TEM) phenotype, potentially delaying CAR-T cell exhaustion. ICOS, part of the CD28 costimulatory molecule family, significantly increased T cell persistence when combined with 4-1BB costimulatory domains in CAR constructs.
Despite conflicting evidence, CD28-CAR is recognized for high effector function and limited T cell persistence, whereas 4-1BB-CAR and ICOS-CAR offer lower potency but longer duration. These observations suggest that costimulatory domain structure significantly affects CAR-T cell persistence.
In Vitro Manipulation and Lymphodepletion
Manufacturing T cells requires obtaining sufficient numbers of healthy T cells from patients. However, post-chemotherapy, especially with treatments containing clofarabine or doxorubicin causing lymphocyte depletion, final CAR-T cell quality may be suboptimal, impacting treatment efficacy.
Normalization of the CD4/CD8 ratio or using naive or memory cell subsets has shown better persistence in preclinical models. Several studies demonstrated that enriching early lineage cell populations yielded better expansion and increased CAR-T cell persistence and efficacy. Adding antioxidants like N-acetylcysteine to cell cultures during manufacturing also inhibited effector differentiation and promoted TSCM cell expansion.
Before CAR T cell infusion, patients undergo lymphodepleting chemotherapy, commonly with cyclophosphamide (cy), fludarabine (flu), and bendamustine (ben). Lymphodepletion eradicates regulatory T cells (Treg) and other immunosuppressive cells, increasing CAR-T cell expansion and persistence. Adequate lymphodepletion is crucial for treatment success and may prevent CAR rejection, as observed in clinical trials for adult B-ALL and B-NHL.
T Cell Exhaustion
In the TME, myeloid-derived suppressor cells (MDSC), cancer-associated fibroblasts (CAF), and tumor cells produce immunosuppressive cytokines causing T cell exhaustion.
Chronic antigen exposure can also lead to T cell exhaustion, with continuous antigen stimulation resulting in epigenetic, metabolic, and transcriptional changes characteristic of exhaustion. This process occurs progressively, with IL-2 and TNF-α lost early, and IFN-γ and chemokine secretion reduced later in exhaustion progression. Although high proliferation capacity is also lost early, exhausted T cells can still proliferate to a limited extent when stimulated in vivo.
Exhausted cells also exhibit high expression of inhibitory receptors such as PD-1, TIM-3, LAG-3, CD160, BTLA, CTLA-4, and TIGIT.
Strategies to Enhance CAR-T Cell Persistence and Efficacy
Cytokine and Receptor Expression
Fourth-generation CAR T cells have recently been developed to counteract the immunosuppressive environment of the TME and overcome immune exhaustion. TRUMKS designs combine the cytotoxic function of CAR-T cells with in situ delivery of immunomodulatory cytokines. Under an inducible system, cytokines are synthesized upon CAR binding to the antigen, acting autocrinely to increase T cell survival and expansion. Cytokines can also act paracrinally to modulate the surrounding environment and interfere with immunosuppressive cytokines in the TME. A range of cytokines, including IL-12, IL-7, IL-15, IL-18, IL-21, and IL-23, are currently under investigation and have entered early clinical trials.
IL-12 is a pro-inflammatory cytokine that induces Th1CD4+ T cell responses, promotes CD8+ clonal expansion and persistence, regulates CTL and NK cell cytotoxic activity, reactivates anergic tumor-infiltrating lymphocytes, recruits NK cells, and inhibits Treg. Preclinical studies on structurally expressed IL-12 in CD19-CAR-T cells showed enhanced tumor-killing effects and immune memory against cancer antigens. However, potential fatal toxicity associated with IL-12 necessitates developing an inducible system to restrict IL-12 secretion only upon CAR activation. Several clinical trials (NCT02498912, NCT03932565, and NCT03542799) are ongoing and recruiting.
IL-15 stimulates CD8+ T cell and NK cell activation, proliferation, and cytotoxic activity. IL-18 increases Th1 cell cytokine production while inhibiting IL-10 synthesis. Both IL-15 and IL-18 are immune response enhancers tested on CAR-T cells. Compared to conventional CAR-T cells, IL-18 and IL-15-secreting CAR-T cells demonstrated enhanced expansion and persistence in tumor-bearing mice and enhanced tumor cytotoxicity in vitro and in vivo. Several clinical trials are currently recruiting to test CARs secreting IL-15 and IL-18 in solid and hematologic tumors.
Recent studies indicate the possibility of co-expressing multiple cytokines in the same CAR-T cell. NCT04833504 is a recently completed clinical trial testing CD19+ CAR-T cells expressing IL-7 and CCL19 in patients with relapsed or refractory B cell lymphoma, with results yet to be reported. Two other clinical trials are currently recruiting to test CAR-T cells expressing IL-7 combined with PD-1 blockers or other cytokine secretions.
Combined Checkpoint Blockade Therapy
Strategies to reduce exhaustion by inhibiting checkpoint signals include using shRNA expression vectors or CRISPR/Cas9 to knock out co-inhibitory molecules. Many studies report that blocking checkpoint inhibition can restore cytokine production and promote CAR-T cell survival. Additionally, simultaneous blockade of multiple immune checkpoints, such as PD-1, TIM-3, and LAG-3, can synergistically enhance CAR-T cell effector function.
Combining CAR-T cells with immune checkpoint blockade therapy could effectively enhance antitumor activity, persistence, and memory cell formation. Anti-PD-1 antibodies enhanced anti-HER2 CAR-T cell antitumor activity in glioblastoma and breast cancer cell lines. However, some clinical trials reported negative results after using PD-1 inhibitors combined with anti-GD2 CAR-T cells in treating neuroblastoma patients.
Additionally, other strategies to block PD-1, such as gene editing to make CAR-T cells secrete PD-1 blocking antibodies or downregulate PD-1, have been employed.
Use of Stem Cell Memory T Cells
Effector T cells were initially considered the best ACT product due to their tumor-killing efficacy. However, they have limited persistence and poor expansion capacity, easily leading to exhaustion.
TSCM cells, with their long lifespan, strong self-renewal capacity, and ability to differentiate into various T cell populations, are ideal candidates for ACT. Clinical studies have shown that infusing phenotypic and functional TSCM-like CAR-T cells (CD62L+, CD28+, and CD27+) produces favorable outcomes. For example, patients with CLL and multiple myeloma treated with anti-CD19 CAR-T cells showed good responses associated with the CD27+ CD45RO− CD8 cell population. Additionally, mouse studies indicated that infusing CD62L+-enriched T cell populations increased expansion and persistence, leading to sustained tumor regression.
Conclusion
Despite significant progress in CAR-T cell therapy over the past decade, limited persistence of
CAR-T cells in patients remains a challenge, primarily due to T cell exhaustion. By optimizing CAR design, altering production conditions, or introducing new therapeutic approaches, we may create engineered T cells resistant to exhaustion, thereby expanding the clinical application of CAR-T cells. While results to date are limited, the potential is vast, and breakthroughs may be imminent.
Factors Influencing CAR-T Cell Persistence and Countermeasures
Reference:
1.Improving CAR T-Cell Persistence. Int J MolSci. 2021 Oct; 22(19): 10828.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



