Strategies to Enhance CAR-T Cell Persistence in Cancer Therapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Strategies to Enhance CAR-T Cell Persistence in Cancer Therapy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Strategies to Enhance CAR-T Cell Persistence in Cancer Therapy
Over the past decade, significant progress has been made in improving the efficacy of CAR-T cell therapy. However, the clinical benefits remain limited, particularly in solid tumors.
Even in hematologic malignancies, patients responding to CAR-T therapy still face the risk of relapse due to factors such as poor T cell expansion and lack of long-term persistence after adoptive transfer.
This challenge is more pronounced in solid tumors, where the tumor microenvironment negatively impacts T cell survival, infiltration, and activity. One hallmark of cancer is the abundance of inhibitory factors in the tumor microenvironment leading to impaired T cell function and exhaustion.
Limited persistence continues to be a major obstacle in the development of CAR-T therapy, encountered from the cell manufacturing steps onwards.
This includes the design of the CAR, in vitro manipulation, and culture conditions, all of which may play crucial roles.
Therefore, engineering CAR-T cells to enhance their survival and reverse or prevent exhaustion phenotypes may be a logical therapeutic approach.
Modern synthetic biology and genome editing technologies provide opportunities, and some studies have explored methods to overcome limitations and create anti-exhaustion T cells, testing them in several key clinical trials.
These approaches may address the persistence issues of CAR-T cells, offering hope for patients.
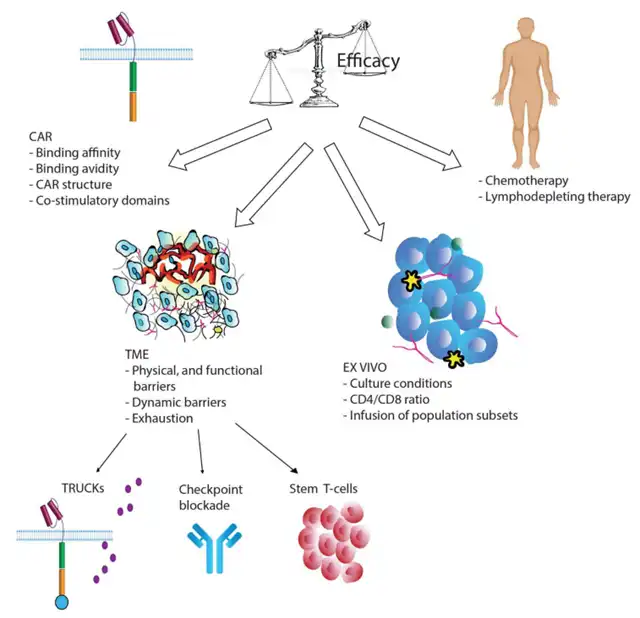
Factors Influencing CAR-T Cell Persistence
1. CAR Design
The type of extracellular CAR domain, the spacer region between the single-chain antibody and the transmembrane domain, and the co-stimulatory domains constituting the CAR have profound effects on T cell function and persistence.
Most CARs used in clinical trials are derived from mice, potentially leading to humoral and CD8+ T cell-mediated immune reactions, resulting in immunological rejection. The reinfusion of CAR-T cells containing mouse components into patients experiencing CD19+ relapse after initial treatment is essentially ineffective. Additionally, the mouse CAR receptor clusters on the cell surface can generate strong signals, leading to T cell exhaustion. In 2016, CAR-T cells with humanized scFv entered clinical trials, showing similar cytotoxic activity to mouse CAR but enhanced persistence due to lower immunogenicity.
CAR affinity influences the strength of signals, and excessive affinity to target cells may lead to T cell exhaustion. A study developed a CAT-CAR (CD19 single-chain antibody) with 40 times lower affinity to CD19 than traditional CARs. Tested in relapsed or refractory pediatric B-ALL patients, clinical studies found sustained responses in 11 out of 14 patients with increased CAR-T cell expansion.
Moreover, co-stimulatory domains play a critical role in the persistence and efficacy of CAR-T cells. Co-stimulatory molecules include CD28, ICOS, CD27, 4-1BB, OX40, and CD40L. Several studies have found enhanced T cell persistence in CAR structures equipped with the 4-1BB domain rather than the CD28 domain. Combining ICOS and 4-1BB co-stimulatory domains in CAR structures significantly increased T cell persistence.
While contradictory evidence exists, there’s a recognition that CD28-CAR is associated with high effector function and limited T cell persistence, whereas 4-1BB-CAR and ICOS-CAR exhibit lower potency but longer duration. Thus, these observations suggest that the structure of co-stimulatory domains is a crucial factor influencing CAR-T cell persistence.
2. In Vitro Manipulation and Lymphodepletion
The process of manufacturing T cells involves obtaining a sufficient number of healthy T cells from the patient. However, lymphocyte reduction due to chemotherapy, especially with agents like chlorambucil or alemtuzumab, leads to suboptimal quality of the final CAR-T cells, and the type of T cells used for infusion significantly affects the treatment’s effectiveness.
Normalizing the CD4/CD8 ratio or using naive or memory cell subpopulations has been shown to achieve better persistence in preclinical models. Studies suggest that using T cell subsets enriched with early lineage cells can improve expansion and enhance the persistence and efficacy of CAR-T cell therapy. Additionally, adding antioxidants like N-acetylcysteine to cell cultures during the manufacturing process can inhibit effector differentiation and promote the expansion of TSCM cells.
In the days leading up to CAR T cell infusion, patients undergo lymphodepleting chemotherapy, most commonly using cyclophosphamide (cy), fludarabine (flu), and bendamustine (ben). Lymphodepletion eradicates regulatory T cells (Treg) and other immunosuppressive cells, increases CAR-T cell expansion, and prolongs their persistence. Adequate lymphodepletion is crucial for treatment success, as observed in clinical trials for adult B-ALL and B-NHL, and it may prevent CAR rejection.
3. T Cell Exhaustion
In the tumor microenvironment (TME), the presence of myeloid-derived suppressor cells (MDSCs), cancer-associated fibroblasts (CAFs), and immune-suppressive cytokines produced by tumor cells leads to T cell exhaustion.
Chronic exposure to antigens can also cause T cell exhaustion. If antigen stimulation persists, T cells undergo a series of epigenetic, metabolic, and transcriptional changes, indicating a state of exhaustion. This process occurs progressively, with early loss of IL-2 and TNF-α, followed by reduced secretion of IFN-γ and chemokines in the later stages of exhaustion. Although high proliferative capacity is lost early, exhausted T cells can still undergo limited proliferation when stimulated in vivo.
Exhausted cells also exhibit high expression of inhibitory receptors, such as PD-1, TIM-3, LAG-3, CD160, BTLA, CTLA-4, and TIGIT.
Strategies to Improve CAR-T Cell Persistence and Efficacy
1. Expression of Cytokines and Their Receptors
Recently developed fourth-generation CAR-T cells aim to resist the immune-suppressive environment in the TME while overcoming immune exhaustion. TRUMKS is designed to combine the cytotoxic function of CAR-T cells with the in-situ delivery of cytokines possessing immune-regulatory capabilities. Under the influence of an inducible system, cytokines are synthesized upon CAR binding to the antigen, exerting their effects in an autocrine manner to enhance T cell survival and expansion. Cytokines can also act in a paracrine manner, modulating the surrounding environment and interfering with immunosuppressive cytokines present in the TME. Several cytokines, including IL-12, IL-7, IL-15, IL-18, IL-21, and IL-23, are currently under research and have entered early clinical trial stages.
IL-12, an inflammatory cytokine, induces Th1CD4+ T cell responses, promotes CD8+ clone expansion and persistence, regulates cytotoxicity in CTLs and natural killer (NK) cells, reactivates exhausted tumor-infiltrating lymphocytes, recruits NK cells, and inhibits Treg. Preclinical studies of CD19-CAR-T cells expressing IL-12 structurally showed enhanced tumor-killing effects and immune memory against cancer antigens. However, the potential lethal toxicity associated with IL-12 makes it necessary to develop an inducible system limiting IL-12 secretion only when CAR is activated. Several clinical studies (NCT02498912, NCT03932565, and NCT03542799) are ongoing and recruiting.
IL-15 is a cytokine that stimulates activation, proliferation, and cytotoxic activity of CD8+ T cells and NK cells. IL-18 increases the production of Th1 cell cytokines while inhibiting IL-10 synthesis. Both IL-15 and IL-18 are enhancers of the immune response and have been tested on CAR-T cells. CAR-T cells secreting IL-18 and IL-15 have shown enhanced expansion and persistence in tumor-bearing mice and demonstrated increased tumor cell cytotoxicity in vitro and in vivo. Multiple clinical trials are recruiting, testing the effects of IL-15 and IL-18 secretion by engineered T cells and NK cells in solid tumors and hematologic malignancies.
Recent studies suggest that co-expressing multiple cytokines in the same CAR-T cell is possible. NCT04833504 is a recently completed clinical trial where CD19+CART cells expressing IL-7 and CCL19 were tested in patients with relapsed or refractory B-cell lymphoma, but results are pending. Two additional clinical trials are recruiting to investigate the combination of CAR-T cells expressing IL-7 with PD-1 blockade or the secretion of other cytokines.
2. Combination Checkpoint Blockade Therapy
Strategies to reduce exhaustion by inhibiting checkpoint signals include expressing shRNA vectors or CRISPR/Cas9 knockout of co-inhibitory molecules. Numerous studies report that blocking checkpoint inhibition can restore cytokine production and promote CAR-T cell survival. Additionally, simultaneously blocking multiple immune checkpoints, such as PD-1, TIM-3, and LAG-3, can synergistically enhance the effector function of CAR-T cells.
Combining CAR-T cells with checkpoint blockade therapy may be an effective strategy to enhance anti-tumor activity, persistence, and memory cell formation. In glioblastoma and breast cancer cell lines, anti-PD-1 antibodies enhanced the anti-tumor activity of HER2 CAR-T cells. However, some clinical trial reports indicate negative results when combining PD-1 inhibitors with anti-GD2 CAR-T cells in treating neuroblastoma patients.
Furthermore, some studies have employed alternative strategies to block PD-1, such as gene editing to enable CAR-T cells to secrete PD-1-blocking antibodies or downregulate PD-1.
3. Use of Stem-Like T Cells
Effector T cells were initially considered the optimal product for ACT because of their efficacy in killing tumor cells. However, their limited persistence and poor expansion capability make them prone to exhaustion.
TSCMs, due to their long lifespan, strong self-renewal ability, and differentiation potential in various T cell subsets, are ideal candidates for ACT. Clinical studies suggest that infusing phenotype and functional TSCM-like CAR-T cells (CD62L+, CD28+, and CD27+) yields favorable outcomes. For example, CLL and multiple myeloma patients treated with CD19 CAR-T cells showed a good response associated with the CD27+CD45RO-CD8 cell subset. Additionally, studies in mice suggest that injecting T cell subsets enriched with CD62L+ cells can increase expansion and persistence, leading to prolonged tumor regression.
Conclusion
Despite significant progress in CAR-T cell therapy over the past decade, the limited persistence of CAR-T cells in patients remains a challenge, primarily due to T cell exhaustion.
By addressing CAR structural design, modifying production conditions, or introducing new therapeutic approaches, engineered T cells resistant to exhaustion may be created, further expanding the clinical applications of CAR-T cells.
Although results so far are limited, the possibilities are immense, and breakthroughs may be on the horizon.
Strategies to Enhance CAR-T Cell Persistence in Cancer Therapy
References:
1.Improving CAR T-Cell Persistence. Int J MolSci. 2021 Oct; 22(19): 10828.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



