Breakthrough in Alzheimer’s Disease: First Late-Onset AD Vaccine Shows Promise
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Breakthrough in Alzheimer’s Disease: First Late-Onset AD Vaccine Shows Promise
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Breakthrough in Alzheimer’s Disease: First Late-Onset AD Vaccine Shows Promise. Clinical trial results reveal significant slowing of Aβ plaque accumulation.
In a groundbreaking development, the first clinical trial data for the prevention of late-onset Alzheimer’s disease (AD) has been released, showing a significant reduction in the rate of Aβ plaque accumulation.
Two effective methods to lower Aβ plaque levels in the brain are the use of anti-β-amyloid (Aβ) monoclonal antibodies and active immunotherapy inducing humoral immune responses. Active immunotherapy involves injecting short or fragmentary Aβ peptides to stimulate the immune system, producing antibodies against Aβ, reducing Aβ plaque deposition, and associated fewer amyloid-related imaging abnormalities (ARIA) [1].
CAD106, a second-generation active immunotherapy based on Aβ, was developed earlier, containing multiple copies of the Aβ1-6 peptide segment to stimulate a robust B-cell response without activating Aβ-specific T-cell responses linked to severe adverse reactions [1-3].
In a phase 2 clinical trial, CAD106 demonstrated good safety and tolerability, with a significant correlation between antibody response and changes in brain amyloid levels [4]. These findings positioned CAD106 as a potential vaccine for Alzheimer’s disease.
Building on these discoveries, the Alzheimer’s Disease Prevention Initiative (API) launched the first late-onset AD prevention clinical trial. Participants with a genetic risk for late-onset AD, but with intact cognition, received preventive treatment based on CAD106.
Recently published in Alzheimer’s & Dementia, the trial results indicate that the majority of participants in the CAD106 group exhibited detectable serum Aβ immunoglobulin G (IgG) titers. In comparison to the placebo group, CAD106 significantly slowed the increase in Aβ plaque formation (average annual change in Aβ PET Centiloid values: -0.91 vs. 8.36) [5].

The study, terminated prematurely on September 23, 2019, unrelated to CAD106, was influenced by negative results from other anti-amyloid treatments. Despite the limited sample size and follow-up duration, researchers believe their findings support larger-scale clinical trials of Aβ active immunotherapy for AD prevention in populations at risk.
The study included 65 cognitively intact participants, with an average age of 65, all APOEε4 homozygotes, randomly assigned to CAD106 450μg + alum 450μg (adjuvant) or placebo + alum in a 5:3 ratio.
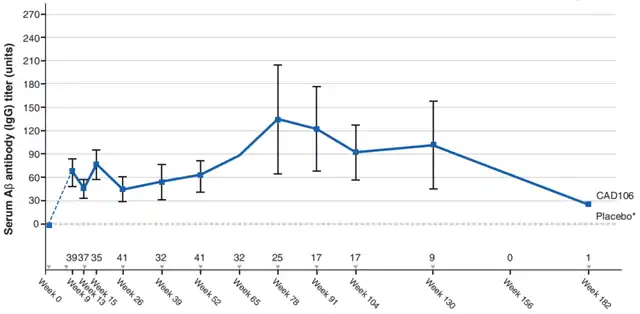
The majority of CAD106 participants received treatment for ≥18 months. Aβ-specific IgG titers were detected only in the CAD106 group, with 97.6% (41/42) exhibiting detectable Aβ-specific IgG titers. Seventeen participants agreed to continue antibody testing every three months post-study completion, and at the end of follow-up (approximately 2 years after the last injection), 82% still had detectable antibody titers.
CAD106 did not activate Aβ-specific T cells.
In PET scans conducted between 18-24 months after treatment, the placebo group exhibited a significantly greater increase in amyloid plaque formation compared to the CAD106 group (average annual change in Aβ PET Centiloid values: -0.91 vs. 8.36, p < 0.001). Exploratory analysis revealed that, regardless of baseline Aβ levels, the CAD106 group showed significantly lower rates of Aβ plaque deposition compared to the placebo group.
After adjusting for baseline Aβ levels, CAD106 had a significant impact on Aβ clearance compared to the placebo.
In the CAD106 group, there was a weak linear relationship between the annual change in Aβ PET results and antibody titers.
Brain volume measurements at weeks 26 and 52 showed no significant differences between the CAD106 and placebo groups. The average changes in cognitive function assessments were also comparable to baseline in both groups.
Overall, 90.5% and 91.3% of participants in the CAD106 and placebo groups, respectively, reported adverse events (AE). The CAD106 group had a higher incidence of general reactions and injection site reactions compared to the placebo group (64.3% vs. 21.7%), with similar rates of other AEs. One participant in the CAD106 group discontinued treatment due to injection-related reactions and sensory abnormalities. Three participants in the CAD106 group had MRI findings consistent with ARIA standards, including one symptomatic ARIA edema and two asymptomatic ARIA hemorrhages.
Except for one serious injection site reaction in the CAD106 group, all suspected drug-related AEs were mild to moderate. No cases of meningitis or encephalitis AEs were reported.
In conclusion, CAD106 demonstrates good safety and tolerability. In APOEε4 homozygotes with intact cognition receiving CAD106 treatment, Aβ antibody titers consistently increased, significantly inhibiting Aβ plaque accumulation. Researchers believe this study provides evidence of the value of Aβ active immunotherapy in primary and/or secondary prevention of AD, supporting further exploration in larger clinical trials.
Breakthrough in Alzheimer’s Disease: First Late-Onset AD Vaccine Shows Promise
References:
[1] Chackerian B, Rangel M, Hunter Z, et al. Virus and virus-like particle-based immunogens for Alzheimer’s disease induce antibody responses against amyloid-β without concomitant T cell responses[J]. Vaccine, 2006, 24(37-39): 6321-6331.
[2] Winblad B, Andreasen N, Minthon L, et al. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study[J]. The Lancet Neurology, 2012, 11(7): 597-604.
[3] Wiessner C, Wiederhold K H, Tissot A C, et al. The second-generation active Aβ immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects[J]. Journal of Neuroscience, 2011, 31(25): 9323-9331.
[4] Vandenberghe R, Riviere M E, Caputo A, et al. Active Aβ immunotherapy CAD106 in Alzheimer’s disease: A phase 2b study[J]. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 2017, 3(1): 10-22.
[5] Riviere ME, Langbaum JB, Turner RS, et al. Effects of the active amyloid beta immunotherapy CAD106 on PET measurements of amyloid plaque deposition in cognitively unimpaired APOE ε4 homozygotes. Alzheimer’s Dement. 2023; 1-12. https://doi.org/10.1002/alz.13532
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



