First Alzheimer’s Disease Gene Silencing Therapy Shows Promise
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
First Global Alzheimer’s Disease Gene Silencing Therapy Shows Promise with Sustained Reduction in Tau Protein Levels Over 25 Months!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
“JAMA Neurology”: First Global Alzheimer’s Disease Gene Silencing Therapy Shows Promise with Sustained Reduction in Tau Protein Levels Over 25 Months!
At the recently concluded 2023 Clinical Trials on Alzheimer’s Disease (CTAD) conference, Biogen presented data from the long-term extension (LTE) phase of the phase 1b clinical trial of their AD drug, BIIB080[1]. Detailed results were published in the “Journal of the American Medical Association – Neurology” on October 30, 2023[2].
BIIB080 is an antisense oligonucleotide (ASO) drug that targets microtubule-associated protein tau (MAPT), selectively reducing MAPT mRNA to decrease tau synthesis.
In April of this year, the primary results of the phase 1b clinical trial of BIIB080 were published in the journal “Nature Medicine.” Mild AD patients in the two high-dose groups saw an average reduction of over 50% in total tau (t-tau) concentration in cerebrospinal fluid after 24 weeks of their last dose, with most adverse events being mild to moderate. The reduction of BIIB080 in cerebrospinal fluid t-tau and phosphorylated tau 181 (p-tau181) was dose-dependent[1].
The data from the LTE phase now show that, with quarterly dosing, the dose-dependent decrease in cerebrospinal fluid t-tau and p-tau181 continued and/or was maintained for the two high-dose groups. At 25 weeks, tau PET scans revealed a reduction in tau aggregation, and at 100 weeks, tau PET scans showed a decrease in tau levels in all evaluated brain regions compared to baseline, with the most significant reduction occurring in the temporal lobe[2].

Data presented at CTAD also indicated that at the 100-week mark, subjects in the high-dose groups showed an improvement trend in overall clinical dementia rating scale sum of boxes (CDR-SB), Mini-Mental State Examination (MMSE) cognitive scale, and Functional Activities Questionnaire (FAQ) assessment[1].
Let’s first review the design of this phase 1b trial. Between August 2017 and February 2020, the research team recruited 102 mild AD patients, with a final enrollment of 46 patients aged 50-74 years. They were randomly assigned to five groups: placebo group (12 patients), 10mg group (6 patients), 30mg group (6 patients), 60mg group (9 patients), and 115mg group (13 patients). The treatment period was 13 weeks, with intrathecal injection as the method of administration, except for the 115mg group, which received dosing every 12 weeks, while the other dose groups received dosing every 4 weeks, with a follow-up period of 24 weeks (MAD phase).
In the LTE phase, 7 subjects from the 60mg and 10 from the 115mg high-dose groups seamlessly continued at the same dosage. Three subjects from the 10mg and 5 from the 30mg groups transitioned to the 60mg dosage in the LTE phase. Four subjects from the placebo group converted to BIIB080 at 60mg or 115mg and entered the LTE phase, with dosing frequency adjusted to every 12 weeks. Five subjects dropped out during the study[1].
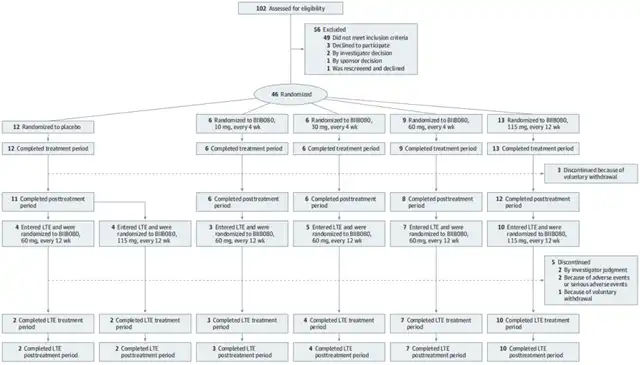
Complete test process
At the end of the MAD phase, which was 24 weeks after the last dose, the 60mg and 115mg groups had an average reduction of 56% and 51% in cerebrospinal fluid t-tau, and a reduction of 56% and 46% in p-tau181, respectively, compared to baseline. This consistent decrease in t-tau and p-tau181 is related to the mechanism of ASO, which can reduce all forms of tau.
In the LTE phase, cerebrospinal fluid t-tau and p-tau181 continued to decrease for the 60mg and 115mg groups, with an average reduction of 67% and 63% in t-tau and 66% and 63% in p-tau181 compared to baseline at the end of the phase. Other groups, including those who transitioned from placebo to BIIB080, showed similar reductions at the end of the LTE phase[2].
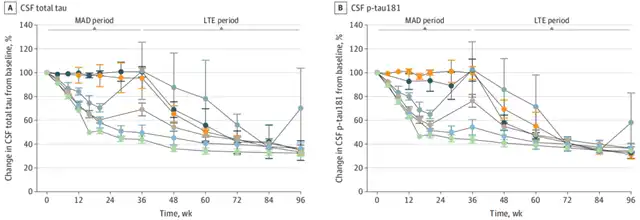
Changes in cerebrospinal fluid t-tau (A) and p-tau181 (B) of subjects during MAD and LTE stages
Longitudinal analysis of individual subjects matched the results obtained at the group level, particularly for the four subjects assigned to the placebo group during the MAD phase who exhibited increased tau aggregation during that phase. This aligns with the natural course of the disease. For those in the two groups initially assigned to 60mg and 115mg, tau levels continued to decrease over the 100-week follow-up period[2].
MRI results indicated that at 25 weeks, the trial drug groups had a larger lateral ventricle volume/total intracranial volume ratio compared to the placebo group. However, both groups experienced a slight decrease in whole brain volume compared to baseline, with no significant difference between the groups. At 100 weeks, the lateral ventricle volume/total intracranial volume ratio continued to increase, and whole brain volume continued to decrease. Additionally, there was a slight reduction in hippocampal volume, but the clinical significance of these changes remains unclear[2].
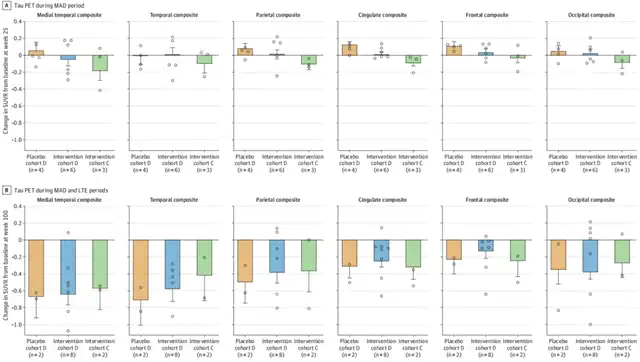
MAD alone (A) and MAD+LTE stage (B), changes in tau levels in 6 brain regions
These results suggest that BIIB080 at doses of 60mg and 115mg can reduce cerebrospinal fluid tau markers in mild AD patients, including t-tau, p-tau181, and tau PET, which are known markers related to cognitive decline. This implies early success for BIIB080. Currently, the phase 2 clinical trial CELIA is recruiting subjects to evaluate the potential of BIIB080 in slowing the progression from mild cognitive impairment to mild dementia caused by AD[1].
Let’s look forward to the future performance of BIIB080 in clinical trials!
Reference:
[1] https://investors.biogen.com/news-releases/news-release-details/new-data-biogens-investigational-antisense-oligonucleotide-aso
[2] https://jamanetwork.com/journals/jamaneurology/article-abstract/2810958
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



