FDA approves restart of Astellas gene therapy AT132 rare disease clinical trial
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA approves restart of Astellas gene therapy AT132 rare disease clinical trial
FDA approves restart of Astellas gene therapy AT132 rare disease clinical trial. The FDA lifted the ban on AT132 for the clinical trial of ASPIRO in patients with X-linked myotubular myopathy (XLMTM).
On December 24, 2020, Audentes Therapeutics, a gene therapy company of Astellas, announced that the FDA has lifted the ban on AT132 for the clinical trial of ASPIRO in patients with X-linked myotubular myopathy (XLMTM).
XLMTM is a serious and life-threatening neuromuscular disease characterized by extreme muscle weakness, respiratory failure and premature death. The mortality rate in the first 18 months of life is expected to be 50%. For patients who survived infancy, a 25% mortality rate is still expected at age 10. XLMTM is caused by a mutation in the MTM1 gene, which results in a deficiency or dysfunction of myostatin, which is necessary for the normal development, maturation and function of skeletal muscle cells. The disease affects approximately 1 in 40,000 to 1 in 50,000 newborn males.
XLMTM brings a huge medical burden to patients, families and healthcare systems. More than 80% of XLMTM patients need ventilator support, and most patients need a gastrostomy tube to provide nutritional support. However, the currently available treatment options are only supportive therapies including ventilator or feeding tube.
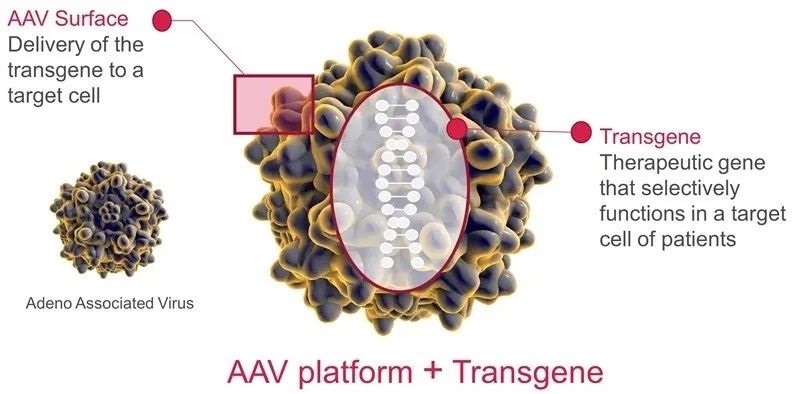
AT132 is loaded with a functional copy of MTM1 gene by AAV8 vector. After a single intravenous administration, AAV8 can deliver normal genes to skeletal muscle, thereby increasing the expression of myostatin in the tissue. AT132 has been granted the FDA Regenerative Medicine Advanced Therapy (RMAT), Rare Pediatric Disease, Fast Track, and Orphan Drug designation, and has been granted priority drug (PRIME) and Orphan Drug designation by the EMA.
ASPIRO is an open-label dose escalation trial designed to evaluate the safety and preliminary effectiveness of AT132 in the treatment of XLMTM patients under 5 years of age. Patients in the experimental group received a dose of 1×1014 vg/kg or 3×1014 vg/kg. The primary endpoints include safety (adverse events and certain laboratory tests) and effectiveness (neuromuscular and respiratory function assessment). Secondary endpoints include disease burden and health-related quality of life, as well as histology and biomarkers of muscle tissue.
In August 2017, the research was officially launched. Subsequently, Audentes published positive test data many times. The preliminary results showed that AT132 can improve the patient’s myosin expression, neuromuscular function, ventilator dependence and respiratory function and other indicators.
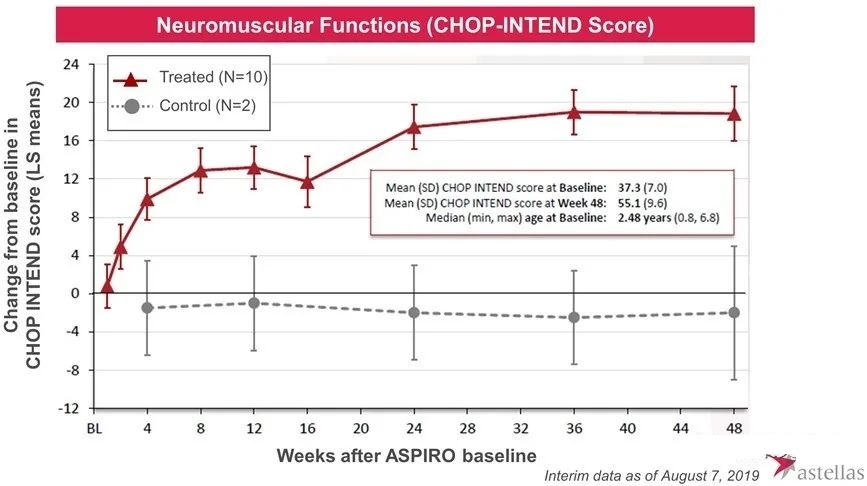
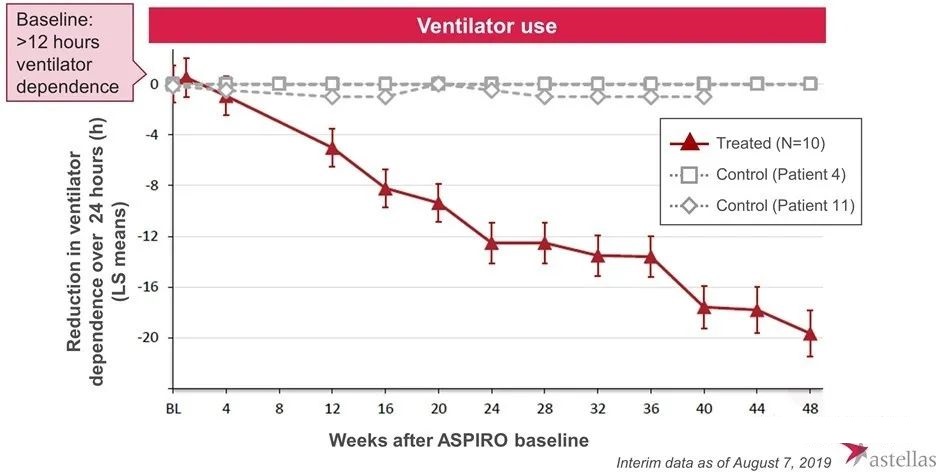
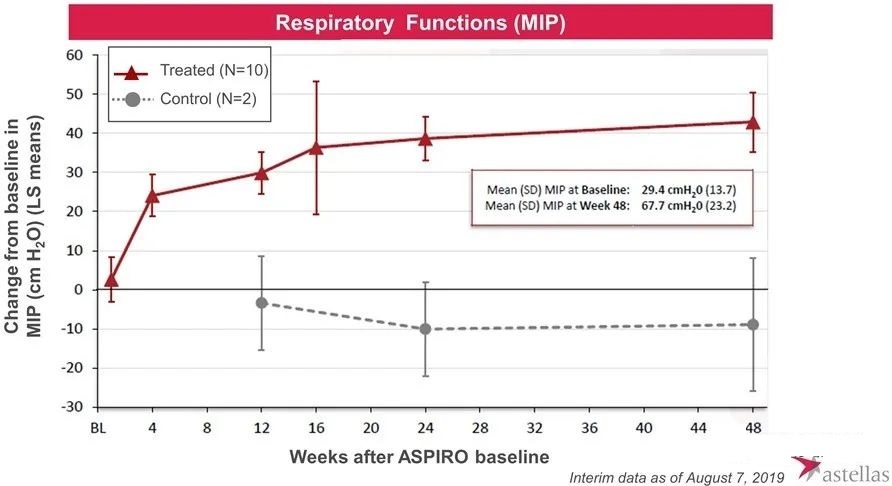
However, in May 2020, one patient who received high-dose AT-132 died of sepsis, and two other patients who received high-dose also experienced serious side effects. In June 2020, one of the two patients also died of bacterial infection and sepsis, which occurred within 4-6 weeks of the 3×1014 vg/kg dose. Therefore, the FDA suspended the trial. In August 2020, the third patient died, and preliminary investigations indicated that the direct cause of death was gastrointestinal bleeding. The patient also received a dose of 3×1014 vg/kg and began to show signs of abnormal liver function within 3-4 weeks after the administration.
All three patients showed evidence of previous hepatobiliary disease, and Audentes pointed out that more than half of the patients who participated in the ASPIRO study had this condition. However, patients who received a dose of 1×1014 vg/kg, or other patients who received a dose of 3×1014 vg/kg, did not experience similar liver function abnormalities. As of August, a total of 23 patients had received AT132 treatment, of which 6 patients had a dose of 1×1014 vg/kg and 17 had a dose of 3×1014 vg/kg.

Currently, Audentes is working hard to complete the clinical and registration related work required to resume drug delivery, and plans to discuss with regulatory agencies in the future to seek to promote the development of AT132 globally. We are also here to take the happiness of Audentes to wish everyone a Merry Christmas!
(source:internet, reference only)
Disclaimer of medicaltrend.org



