DMD gene therapy phase 2 clinical trials are not effective
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
DMD gene therapy phase 2 clinical trials are not effective
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
DMD gene therapy phase 2 clinical trials are not effective, Sarepta’s stock price cuts down.
The Dystrophin gene is very large, with as many as 79 exons, so direct delivery of the correctly encoded Dystrophin gene is not feasible.
Updated news:
The first DMD gene therapy SRP-9001 may cost 4 million US dollars
Duchenne muscular dystrophy (Duchenne muscular dystrophy, DMD) is a recessive genetic disease of the X chromosome, so it mainly affects boys. According to statistics, on average, one out of every 3,500-5,000 newborn boys in the world suffers from this disease.
Patients generally start to develop symptoms at the age of 3-5, and they first show progressive leg muscle weakness, which causes inconvenience to walk. It usually loses the ability to walk at the age of 12, heart and breathing weakness begin in adolescence, and cause serious complications, usually at the age of 20-30 years old due to respiratory failure and death.
On January 8, 2020, Sarepta, a leading company in the treatment of Duchenne muscular dystrophy affected by the poor results of Phase 2 clinical trials, the company’s share price was cut in half and plummeted by more than 50%.
Duchenne muscular dystrophy (DMD) is a single-gene disease with a relatively high incidence. The mutation of the gene encoding dystrophin (Dystrophin) on the X chromosome results in the inability to produce enough dystrophin. The patient’s muscle tissue Gradually replaced by fat and fibrotic tissue.
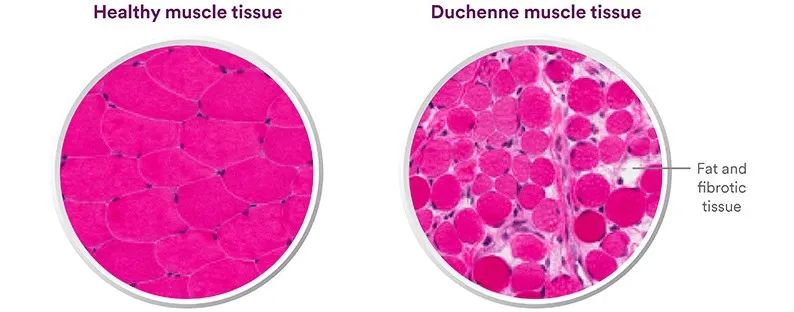
However, the Dystrophin gene is very large, with as many as 79 exons, so direct delivery of the correctly encoded Dystrophin gene is not feasible.
Therefore, there are currently two main treatment strategies. One method is to edit the DNA or mRNA of the Dystrophin gene, skip the exons with mutation sites, and encode a shortened dystrophin that is still functional.
Another method is to extract part of the Dystrophin gene to create a smaller micro-Dystrophin gene (micro-Dystrophin), which is still functional and small enough to be delivered using AAV viral vectors.
Sarepta is conducting research on these two treatment strategies. Among them, the SRP-9001 based micro-Dystrophin strategy is more advanced.
On January 8, 2021, Sarepta’s SRP-9001 gene therapy for the treatment of Duchenne muscular dystrophy was not effective in phase 2 clinical trials. Duchenne muscular dystrophy treatment is Sarepta’s most important project. Affected by this, Sarepta’s stock price fell by more than 50%.
In this phase 2 clinical trial, 41 patients with Duchenne muscular dystrophy aged 4 to 7 years were randomly divided into two groups receiving SRP-9001 treatment or receiving placebo treatment. After 48 weeks, the patient was tested for exercise ability, and the results of the treatment group were not better than the placebo group.
In the first half of 2020, Pfizer’s Duchenne muscular dystrophy treatment started a global multi-center phase 3 clinical trial. Pfizer adopted the same strategy as Sarepta’s SRP-9001 gene therapy, through AAV intravenous injection of mini dystrophin gene (Mini-Dystrophin), Pfizer has recently completed the first patient administration.
Compared with Sarepta’s SRP-9001 gene therapy, Pfizer’s gene therapy is not as safe as the former, but the phase 2 clinical failure of SRP-9001 gene therapy may benefit Pfizer and may cause Pfizer’s Duchenne muscular dystrophy The therapy is listed first.
The first DMD gene therapy SRP-9001 may cost 4 million US dollars
DMD gene therapy phase 2 clinical trials are not effective
(sourcechinanet, reference only)
Disclaimer of medicaltrend.org




3 thoughts on “DMD gene therapy phase 2 clinical trials are not effective”
Comments are closed.