Biogen released Long-Term Clinical Results of Antibody Therapy for Alzheimer’s Disease
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Biogen released Long-Term Clinical Results of Antibody Therapy for Alzheimer’s Disease
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Biogen released Long-Term Clinical Results of Antibody Therapy for Alzheimer’s Disease
On March 17, Biogen announced that the latest data showed that after nearly two and a half years (128 weeks) of treatment with the anti-amyloid beta (Aβ) monoclonal antibody Aduhelm (aducanumab-avwa), two Phase 3 The key pathological features of Alzheimer’s disease — Aβ deposition and plasma p-tau181 levels — consistently decreased significantly in patients in the clinical trial.
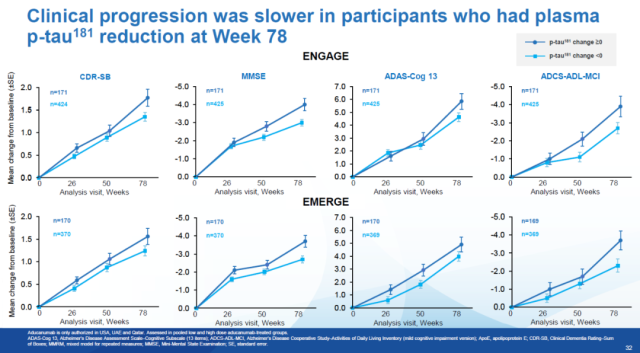
At 78 weeks of treatment, patients with reduced plasma p-tau181 levels had less cognitive decline than patients with no change in p-tau181 levels.
The company also announced that Aduhelm’s Phase 3 clinical trial data were published in The Journal of Prevention of Alzheimer’s Disease.
Aduhelm is an anti-Aβ monoclonal antibody jointly developed by Biogen and Eisai, which promotes the elimination of Aβ deposits in the brain by binding to Aβ in Aβ deposits. It received accelerated approval from the U.S. FDA last year for the treatment of patients with early-stage Alzheimer’s disease.
The data reported this time includes open-label, extended-phase data from two Phase 3 clinical trials, EMERGE and ENGAGE. The data showed that Aduhelm continued to reduce Aβ levels in the patients’ brains after two years of treatment.
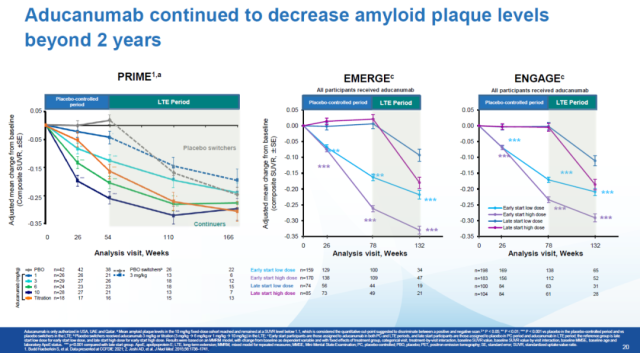 Image source: Biogen official website
Image source: Biogen official website
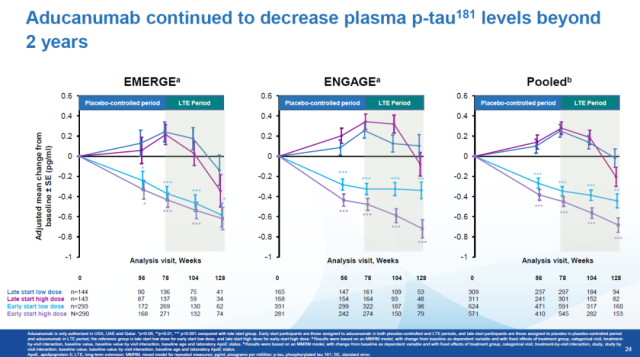
In addition, Aduhelm continued to reduce plasma p-tau181 levels in patients after two years of treatment. Patients with more effective clearance of Aβ deposits also had greater reductions in p-tau181 levels at 128 weeks.
In two phase 3 clinical trials, patients with reduced plasma p-tau181 levels after 78 weeks of treatment, as assessed by 4 different assays (CDR-SB, MMSE, ADAS-Cog13, and ADCS-ADL-MCI) Disease progression is also slower.
References:
[1] Biogen Announces Peer-Reviewed Publication of ADUHELM® Phase 3 EMERGE and ENGAGE Data in The Journal of Prevention of Alzheimer’s Disease. Retrieved March 16, 2022, from https://investors.biogen.com/news-releases/news-release-details/biogen-announces-peer-reviewed-publication-aduhelmr-phase-3
[2] Long-Term Phase 3 Data Show ADUHELM® Continues to Reduce Underlying Pathologies of Alzheimer’s Disease in Patients Treated for More Than Two Years. Retrieved March 16, 2022, from https://investors.biogen.com/news-releases/news-release-details/long-term-phase-3-data-show-aduhelmr-continues-reduce-underlying
[3] Key Milestones in Alzheimer’s Disease. Retrieved March 16, 2022, from https://investors.biogen.com/static-files/3cce2949-6846-45b0-b34c-1187445cfd9d
Biogen released Long-Term Clinical Results of Antibody Therapy for Alzheimer’s Disease
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



