What is the Future of Next-Generation CAR-T Therapy?
- CDC Recommends Updated COVID-19 Vaccines for 2024-2025 Season
- Will China and India produce cheaper “Miracle Weight Loss Drug”Semaglutide soon?
- Keto Diet Accelerates Aging and Promotes Cancer Metastasis
- The Critical Role of Immune Cell Triumvirates in Enhancing CD8+ T Cell Function
- Chinese-made Drug Enters Australia: Causing at Least 20 Deaths!
- UV Rays Threaten Golfers’ Health: Sun Protection Strategies You Need to Know
What is the Future of Next-Generation CAR-T Therapy?
- Chinese-made Drug Enters Australia: Causing at Least 20 Deaths!
- How serious is Japan’s “flesh-eating bacteria” problem?
- Taiwan 6th wave of COVID outbreak: 623 confirmed cases in one week and 38 deaths
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
What is the Future of Next-Generation CAR-T Therapy?
In recent years, Chimeric Antigen Receptor (CAR) T-cell therapy has emerged as a significant breakthrough in treating hematological malignancies.
To date, the U.S. Food and Drug Administration (FDA) has approved six CAR-T therapies for the treatment of non-Hodgkin lymphoma, B-cell acute lymphoblastic leukemia, and multiple myeloma.
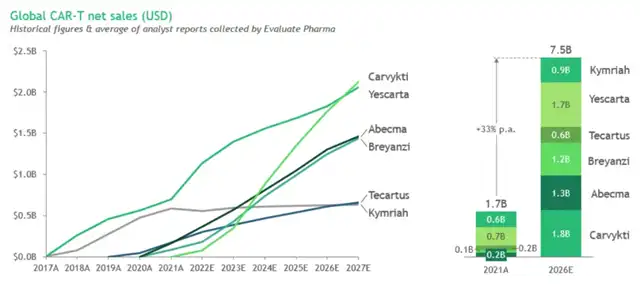
Since the introduction of Kymriah in 2017, the CAR-T market has grown to $1.7 billion in 2021, with sales projected to reach $7.5 billion by 2026, reflecting an annual growth rate of about 33%.
By 2033, the use of CAR-T therapies is expected to increase by 10%, with research focusing on developing next-generation CAR-T therapies.
Currently, most registered clinical trials are based on so-called second-generation CARs, consisting of an extracellular antigen-binding domain, a co-stimulatory domain, and a CD3z signaling domain.
Despite the significant overall therapeutic effects, a considerable proportion of patients do not benefit from CAR-T therapy, with overall response rates ranging from 50% to 100%, and high relapse rates of up to 66% due to limited response durability. Moreover, CAR-T therapy is associated with adverse reactions such as cytokine release syndrome and neurotoxicity.
Advances in immunology and molecular engineering have led to the construction of new-generation CAR-T cells, equipped with various molecular mechanisms, including additional co-stimulatory domains, safety switches, immune checkpoint regulation, cytokine expression, or the knockout of therapeutic interfering molecules. These advancements aim to overcome current limitations of CAR-T therapy, enhance efficacy, and reduce unwanted side effects. Numerous clinical trials are currently evaluating the safety and efficacy of novel CAR-T therapies.
Next-Generation CAR-T Cell Therapy
The innovative prospects of CAR-T therapy can be divided into two directions: cell sources and engineering approaches. Continuous improvements, such as the shift from autologous cells derived from patients to more scalable allogeneic therapy approaches and eventually in situ delivery, may help address limitations related to manufacturing, management, safety, and response durability. Meanwhile, applying gene editing and synthetic biology tools to control and adjust the duration, site, and intensity of CAR-T therapy could improve its safety and effectiveness.
CAR-T Cells Armed with Immune Checkpoint Regulation
Immune checkpoint regulation in CAR-T therapy aims to bypass the suppressive tumor microenvironment. In hematological malignancies, all clinical trials evaluating this approach explicitly rely on disrupting the programmed cell death protein 1 (PD-1) pathway. Although the number of trials is relatively small (n=8), individual studies have demonstrated unique methods for modulating PD-1 signaling.
TRUCKs – CAR-T Cells Expressing Cytokines
T-cells redirected for universal cytokine killing (TRUCKs) are next-generation CAR-T cells designed to express certain cytokines to enhance their antitumor efficacy, improve persistence, and alter the characteristics of the tumor microenvironment.
CAR-T Cells with Safety Switches
The treatment-related toxicity associated with conventional CAR-T therapy has driven the development of CAR-T cells with safety switches. New technologies enable researchers to incorporate safety switches, which can clear CAR-T cells by inducing apoptosis, complement-dependent cytotoxicity (CDC), or antibody-dependent cellular cytotoxicity (ADCC) upon the administration of exogenous drugs.
Universal CAR-T Cells
Traditional CAR-T products are manufactured using autologous T cells from patients eligible for treatment. This approach has several limitations, including long manufacturing times, difficulty mobilizing an adequate number of T cells, and reduced quality of T cells from severely treated patients. However, producing allogeneic CAR-T cells through molecular engineering can overcome these obstacles. Constructing universal CAR-T cells requires removing major histocompatibility complex (MHC) and T-cell receptor (TCR) molecules from donor-derived cells.
Clinical Prospects of Next-Generation CAR-T
Analyzing the current global CAR-T clinical pipeline reveals that while most still utilize traditional single-antigen autologous methods, more next-generation CAR-T therapies are entering clinical trials.
Safety
Safety on/off, switchable, and logic-gated platforms can support improved safety and expand the applicability of CAR-T therapy to more patients. Incorporating truncated epidermal growth factor receptor (EGFRt) into CAR-T cells is a common safety switch method in clinical trials. EGFRt is targeted by cetuximab, which can clear CAR-T cells via CDC or ADCC. As of August 2022, 20 clinical trials are investigating EGFRt-based CAR-T cells in various hematological malignancies. Currently, no data are available on initiating the EGFRt safety switch mechanism in humans, possibly due to the lack of life-threatening adverse events during the investigation period.
The RQR8 suicide gene is a similar method for controlling CAR-T cells after treatment. The RQR8 gene encodes a cell surface protein derived from CD20 and CD34 antigen epitopes. This strategy enables CAR-T cells to be cleared via CDC or ADCC upon rituximab administration. Currently, the NCT03590574 clinical trial is studying RQR8-based CAR-T cells in peripheral T-cell lymphoma (PTCL), with preliminary results showing an overall response rate (ORR) of 67% and a complete metabolic response (CMR) in 56% of patients. Another trial, NCT03287804, was terminated due to suboptimal initial efficacy in treating multiple myeloma.
Adding inducible caspase 9 (iCasp9) is a unique method for CAR-T safety switches. After administering AP1903, the specially modified caspase 9 dimerizes and triggers the apoptosis pathway. Currently, 17 clinical trials are evaluating iCasp9-based CAR-T cells, but only four have provided initial data. The NCT03016377 trial reported a case of neurotoxicity in an acute lymphoblastic leukemia (ALL) patient after CAR-T infusion, with complete symptom relief following AP1903 administration. The only adverse event was grade 2 bilirubin elevation for three days. Interestingly, despite the elimination of over 90% of CAR-T cells, researchers observed significant clinical anti-leukemia responses. The NCT02274584 trial reported a case of Hodgkin lymphoma with temporary partial remission. Additionally, the NCT03125577 trial provided data on four patients, all of whom achieved complete remission (CR) after CAR-T infusion.
Efficacy
Armed CAR-T cells can improve cell homing and infiltration into solid tumors and potentially overcome immunosuppressive tumor microenvironments. Similarly, multi-target CAR-T cells address receptor heterogeneity and antigen escape challenges, offering broader tumor coverage and potentially more durable antitumor activity. For example, Gracell’s GC012F is an autologous BCMA/CD19 dual-target CAR-T therapy currently in an Ib/II phase trial for high-risk relapsed/refractory multiple myeloma. Latest data from the 2023 American Society of Clinical Oncology (ASCO) meeting showed an ORR of 93% and a residual disease-negative rate of 100% in 29 patients, with a median progression-free survival of 38 months and deep and lasting responses.
Universality
Allogeneic CAR-T cells can simplify manufacturing and administration processes, reducing costs and increasing the number of eligible patients. However, safety remains a concern for allogeneic approaches, particularly the risk of graft-versus-host disease (GvHD). Caribou’s CB-010 is an allogeneic anti-CD19 CAR-T therapy that reduces GvHD risk by knocking out the T-cell receptor α gene. In the latest progress of the ANTLER I phase trial, CB-010 showed an overall response rate of 94% and a 6-month complete response rate (CRR) of 44% in 16 relapsed/refractory B-cell non-Hodgkin lymphoma patients. The therapy was well-tolerated, with no grade 3 or higher CRS or GvHD, comparable to the safety of good autologous CAR-T products.
Conclusion
With the accumulation of research data and widespread application of molecular engineering, next-generation CAR-T cell therapies are gradually maturing in clinical settings.
The most successful next-generation CAR-T cells are likely to be universal allogeneic CAR-T cells (manufactured using CRISPR/Cas9 technology), characterized by immune checkpoint resistance and expressing cytokines that transport T cells to tumor sites.
Additionally, this structure will include safety switch mechanisms to manage potential toxicities. This combination could overcome current limitations of CAR-T cell therapy and improve patient outcomes.
What is the Future of Next-Generation CAR-T Therapy?
References
- Next generations of CAR-T cells – new therapeutic opportunities in hematology? Front Immunol. 2022 Oct 28;13:1034707.
- The next-generation CAR-T therapy landscape. Nat Rev Drug Discov. 2023 Sep 6.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



