Overview of the Clinical Landscape of the Next Generation CAR-T Cell Therapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Overview of the Clinical Landscape of the Next Generation CAR-T Cell Therapy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Overview of the Clinical Landscape of the Next Generation CAR-T Cell Therapy
In recent years, chimeric antigen receptor (CAR) T-cell therapy for treating hematologic malignancies has marked a significant breakthrough in cell therapy.
So far, the U.S. Food and Drug Administration (FDA) has approved six CAR-T therapies for non-Hodgkin lymphoma, B-cell acute lymphoblastic leukemia, and multiple myeloma.
Currently, most registered clinical trials are based on the so-called second-generation CAR, consisting of an extracellular antigen-binding domain, a co-stimulatory domain, and a CD3z signaling domain.
Unfortunately, despite a notable overall treatment effect, a considerable proportion of patients still do not benefit significantly from CAR-T therapy, with an overall response rate ranging from 50% to 100%.
The limited durability of responses and a subsequent relapse rate of up to 66% are also observed.
Additionally, adverse reactions such as cytokine release syndrome and neurotoxicity are associated with this therapy.
Advancements in immunology and molecular engineering have led to the development of the next generation of CAR-T cells, armed with various molecular mechanisms including additional co-stimulatory domains, safety switches, immune checkpoint modulation, cytokine expression, or knockout of interfering molecules.
These innovations aim to overcome the current limitations of CAR-T therapy, enhance efficacy, and reduce unnecessary side effects.
Currently, numerous clinical trials are evaluating the safety and efficacy of these novel CAR-T therapies.
Structural Evolution of CARs
As CAR-T cell technology matures, the composition of CAR structures, particularly the intracellular domains, continues to evolve. The first-generation CAR-T cell structure comprises a high-affinity single-chain antibody (scFv) targeting tumor-associated antigens, a transmembrane domain, and a unique intracellular TCR-ζ or FcR-γ domain. However, the clinical benefits of first-generation CARs are limited due to their low proliferative capacity and limited in vivo cytotoxicity.
To overcome these limitations, second-generation CAR structures incorporate additional intracellular co-stimulatory domains, most notably CD28 or TNFRSF member 4-1BB. These CARs have proven to be highly effective, and several CAR-T products have gained regulatory approval for treating hematologic malignancies.
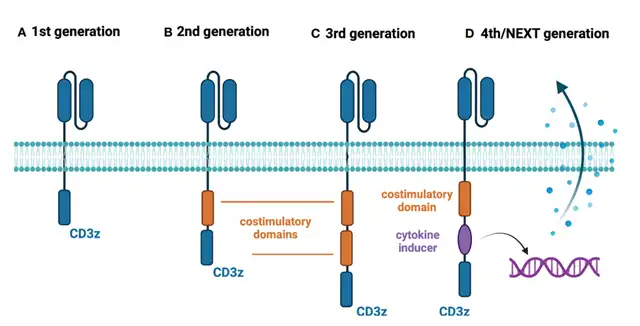
The third-generation CAR-T structure includes a combination of co-stimulatory domains, while fourth and fifth-generation CAR-T cells incorporate additional co-stimulatory domains that induce cytokine expression during antigen recognition or contain intracellular domains of certain cytokine receptors (e.g., truncated IL-2 receptor β chain and STAT3-binding portion).
Arming CAR-T Cells with Immune Checkpoint Modulation
Immune checkpoint modulation in CAR-T therapy aims to overcome the inhibitory tumor microenvironment. In the context of hematologic malignancies, all clinical trials assessing this approach explicitly rely on targeting the programmed cell death protein 1 (PD-1) pathway. One innovative approach in the NCT03258047 clinical trial involves fusing PD-1 to the intracellular CD28 activation domain. This converts the binding of PD-L1 and PD-1 into an activating signal, expected to produce a more effective anti-tumor effect. The study included 17 subjects with B-cell non-Hodgkin lymphoma, and the results showed complete response (CR) and overall response rate (ORR) of 41.2% and 58.8%, respectively. While not demonstrating superiority to second-generation CAR-T therapy for relapsed/refractory diffuse large B-cell lymphoma (CR 52%, ORR 72%), the trial using the PD-1 fusion approach is ongoing in the NCT03932955 clinical trial with no results available yet.
Another exploration to disrupt the PD-1 signal involves reprogramming CAR-T cells to secrete PD-1-Fc fusion protein. Currently, two studies are evaluating the safety and efficacy of this approach in relapsed/refractory multiple myeloma (NCT04162119) and relapsed/refractory B-cell lymphoma (NCT04163302). However, no clinical data has been published at this time.
In the NCT04836507 clinical trial, researchers tested anbalcabtagene autoleucel (Anbal-cel) in patients with relapsed/refractory large B-cell lymphoma (LBCL). This novel CAR-T cell product eliminates PD-1 and TIGIT receptors. Anbal-cel demonstrated impressive results with a CR of 78%. Additionally, PD-1 knockout is being evaluated in the NCT03208556 clinical trial.
Apart from PD-1 pathway modulation, the NCT04037566 clinical trial is assessing another armed CAR-T cell construct. CAR-T cells with reduced expression of hematopoietic progenitor kinase 1 (HPK1)—a negative intracellular immune checkpoint—showed promising preliminary results. In all enrolled patients, 72.7% achieved CR or CRi, comparable to FDA-approved anti-CD19 therapies.
TRUCKs – CAR-T Cells Expressing Cytokines
T cells redirected for universal cytokine-mediated killing (TRUCKs) represent a new generation of CAR-T cells designed to express certain cytokines to enhance the anti-tumor efficacy, improve persistence, and alter the characteristics of the tumor microenvironment.
Currently, six clinical trials are evaluating TRUCKs for treating hematologic malignancies. Four trials (NCT04381741, NCT03929107, NCT04833504, NCT03778346) are assessing CAR-T cells expressing IL-7 and the chemokine CCL19. IL-7 promotes T cell proliferation and survival, while CCL19 guides CAR-T cells to the tumor site. Results show a CR rate of 4/7 and an ORR of 5/7 in patients with relapsed/refractory DLBCL. Moreover, NCT03778346 explores the same approach in patients with relapsed/refractory multiple myeloma (MM), where two selected patients achieved CR (100%).
CAR-T cells expressing the cytokine IL-18 are being evaluated in the NCT04684563 clinical trial. IL-18 expression in CAR-T cells enhances proliferation and anti-tumor activity. However, long-term safety outcomes of IL-18-expressing CAR-T cells in clinical settings remain to be observed.
The NCT03602157 clinical trial represents a different approach in utilizing cytokine signaling in CAR-T cells. Researchers engineered CAR-T cells expressing CCR4 for targeting CD30 in the treatment of relapsed/refractory large B-cell lymphoma (LBCL) and cutaneous T-cell lymphoma (CTCL). This receptor binds to CCL17, thereby improving CAR-T cell trafficking to tumor sites. Preliminary results from the Hodgkin lymphoma cohort were optimistic, with 6 enrolled patients achieving CR (75%), and all patients achieving ORR (100%). Unfortunately, no patients in the CTCL group achieved remission.
Safety Switches in CAR-T Cells
The treatment-related toxicities associated with conventional CAR-T therapy have prompted the development of safety switch CAR-T cells. New technologies enable researchers to incorporate safety switches that induce cell apoptosis, complement-dependent cytotoxicity (CDC), or antibody-dependent cellular cytotoxicity (ADCC) after administering external drugs to clear CAR-T cells.
A common safety switch method in clinical trials is the inclusion of a truncated epidermal growth factor receptor (EGFRt) into CAR-T cells. EGFRt is targeted by cetuximab
, which can eliminate CAR-T cells through CDC or ADCC. As of August 2022, 20 clinical trials are investigating EGFRt-based CAR-T cells in various hematologic malignancies. Currently, there is no available data on the activation of the EGFRt safety switch mechanism in humans, likely due to the absence of life-threatening adverse events during the investigation period.
In terms of efficacy, clinical trials have shown varied preliminary results in CD19-positive malignancies. NCT03085173 reported an overall CR rate of 57%, with DLBCL patients achieving a CR rate of 88% and CLL patients at 22%. Trials targeting B-NHL patients, such as NCT02706405 (42% CR), NCT02153580 (45% CR), and NCT01815749 (75% CR), have reported different response rates.
The suicide gene RQR8 is another approach to control CAR-T cells after treatment. The RQR8 gene encodes a cell surface protein derived from the CD20 and CD34 antigens. This strategy allows for the clearance of CAR-T cells through CDC or ADCC after administering rituximab. Currently, the NCT03590574 clinical trial is investigating RQR8-based CAR-T cells in peripheral T-cell lymphoma (PTCL). Preliminary results show an ORR of 67%, with 56% of patients achieving complete metabolic response (CMR). Another trial (NCT03287804) targeting multiple myeloma was terminated due to suboptimal preliminary efficacy.
The addition of inducible caspase 9 (iCasp9) is a unique method for a safety switch in CAR-T cells. After administering AP1903, specifically modified caspase 9 dimerizes and triggers the apoptosis pathway. Currently, 17 clinical trials are evaluating iCasp9-based CAR-T cells, with only 4 providing initial data. The NCT03016377 clinical trial reported a case of neurotoxicity in an ALL patient after CAR-T infusion. Symptoms completely resolved after administering AP1903, with the only adverse event being a transient 2-grade increase in bilirubin lasting three days. Interestingly, despite eliminating over 90% of CAR-T cells, researchers observed significant clinical anti-leukemia responses. In the NCT02274584 trial, only one case report in a Hodgkin lymphoma patient has been published, showing temporary partial remission. Additionally, the NCT03125577 trial reported data from four patients, all achieving CR after CAR-T infusion.
Another suicide gene that can serve as a safety switch for CAR-T therapy is the herpes simplex virus thymidine kinase (HSV-TK) Mut 2 gene, whose product is targeted by the prodrug ganciclovir (GCV). The terminated NCT04097301 trial evaluated HSV-TK-based CAR-T cells. Ultimately, only two patients were enrolled, and there was no response to treatment. As the safety switch was not activated due to the lack of T cell-related toxicity, further investigation is needed.
Universal CAR-T Cells
Current traditional CAR-T products are manufactured from autologous T cells obtained from eligible patients. This approach has several limitations, including long manufacturing times, difficulty mobilizing an adequate quantity of T cells, and a significant reduction in T cell quality for severe cases. However, molecular engineering has enabled the generation of allogeneic CAR-T cells to overcome these obstacles. To construct universal CAR-T cells, major histocompatibility complex (MHC) and T-cell receptor (TCR) molecules are removed from cells obtained from donor sources.
The preliminary safety and feasibility of CRISPR/Cas9 technology in T cell engineering have been confirmed in the first-in-human trial (NCT03399448). As of August 2022, five clinical trials evaluating anti-self-killing CAR-T cells have been registered on ClinicalTrials.gov. In the NCT04502446 trial, CAR-T cells targeting the CD70 antigen were designed to eliminate the expression of TCR, MHC-I, and CD70 to prevent self-killing and enhance efficacy. Results showed an ORR of 47%, with a CR of 20%, and no severe adverse events were observed. Furthermore, the NCT04264078 trial provided data on the use of universal CAR-T cells in patients with relapsed/refractory T-cell acute lymphoblastic leukemia (T-ALL). Using CRISPR/Cas9 to disrupt TCR and CD7 genes to prevent graft-versus-host disease (GvHD) and self-killing, the results showed a CR of 80% (4/5). However, all subjects experienced severe cytokine release syndrome (CRS) related to treatment (4 patients at grade 3, 1 patient at grade 4). Subsequent results from additional enrolled patients confirmed the high efficacy of the treatment, with a CR of 83% (5/6), and safety outcomes consistent with previous observations.
Conclusion: Overview of the Clinical Landscape of the Next Generation CAR-T Cell Therapy
With the accumulation of research data and widespread application of molecular engineering, the next generation of CAR-T cell therapy is gradually maturing in the clinical setting.
The most successful next-generation CAR-T cells may be universal allogeneic CAR-T cells (manufactured using CRISPR/Cas9 technology), characterized by immune checkpoint resistance and the expression of cytokines to transport T cells to tumor sites.
Additionally, this structure will include safety switch mechanisms for managing potential toxicity.
This combination has the potential to overcome the current limitations of CAR-T cell therapy and contribute to improving patient treatment outcomes.
Overview of the Clinical Landscape of the Next Generation CAR-T Cell Therapy
References:
1.Next generations of CAR-T cells – new therapeutic opportunities in hematology? Front Immunol.2022 Oct 28;13:1034707.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



