3-negative breast cancer: This antibody conjugate with 95% control rate
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
3-negative breast cancer: This innovative antibody conjugate has a disease control rate of 95%!
3-negative breast cancer: This antibody conjugate with 95% control rate. Among 21 evaluable triple-negative breast cancer patients, Dato-Dxd achieved an overall response rate of 43% and a disease control rate of 95%.
During the ESMO Breast Cancer Conference in 2021, AstraZeneca/Daiichi Sankyo developed a new drug DS-1062 (Datopotamab Deruxtecan, Dato-Dxd)-a DXd antibody drug conjugate directed by TROP2, in the field of breast cancer The latest research data is freshly released, and the data is eye-catching.
Among 21 evaluable triple-negative breast cancer patients, Dato-Dxd achieved an overall response rate of 43% and a disease control rate of 95%.

Three-negative Breast Cancer: Much Hard in Breast Cancer treatment
Breast cancer is one of the most common malignant tumors in women. Approximately 1.4 million women worldwide develop breast cancer every year, and half a million women die of breast cancer. In the breast cancer family, there is a “cancer bully”-triple-negative breast cancer, which makes the patient feel painful and helpless. In fact, breast cancer is not what we think, it is just a single type of cancer. In fact, breast cancer is divided into four subtypes: cavity surface A, cavity surface B, HER-2 positive and triple negative, among which triple negative breast cancer is the most “toxic”.
Triple-negative breast cancer refers to breast cancer in which the three main therapeutic targets of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (Her2) are negative. Among all breast cancers, triple-negative breast cancer accounts for 10% to 15%, and it has the characteristics of poor prognosis, strong drug resistance, high recurrence rate, and few treatments, which has become a major problem in the breast industry.
It is estimated that only 12.2% of patients with metastatic triple-negative breast cancer survive for five years, and the median overall survival is generally less than two years.
The disease control rate is as high as 95%, and the new ADC drug targeting the TROP2 target has lived up to expectations!
In this phase 1 study from TROPION-PanTumor01, the patients enrolled are all suffering from advanced or metastatic HR-/HER2- triple-negative breast cancer and have received at least one standard treatment for recurrence or progression.
As of January 8, 2021, of the 21 evaluable patients who received DS-1062 [6mg/kg (n=19) or 8mg/kg (n=2)] through the blind independent center evaluation, the initial total The response rate (ORR) was 43%. Five confirmed complete or partial remissions (CR/PR) were observed, and four were waiting to be confirmed, and the disease control rate was as high as 95%.
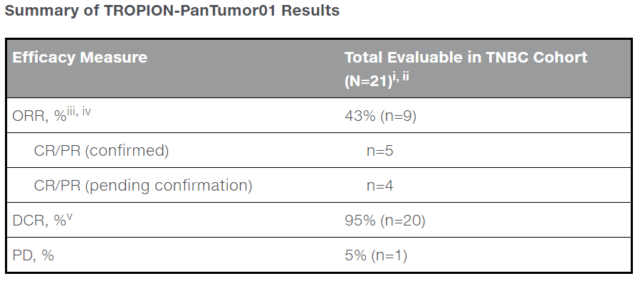
The safety of datopotamab deruxtecan seen in the TNBC cohort is consistent with the safety previously reported in the TROPION-PanTumor01 non-small cell lung cancer (NSCLC) cohort. No patients discontinued treatment due to adverse events (AEs).
Aditya Bardia, MD, Director of Breast Cancer Research at the Massachusetts General Cancer Center, said: “For patients with metastatic triple-negative breast cancer who have previously been treated, current treatments are limited. Historically speaking, this is a very difficult one. The subtype of breast cancer treated.” “The initial safety and effectiveness of DS-1062 in triple-negative breast cancer patients is encouraging and provides further development for breast cancer patients.”
Target the four major cancer types! Broad-spectrum ace new drug DS-8201 is in full swing
Just after talking about DS-1062, I have to mention its predecessor, the famous broad-spectrum ace ADC drug targeting the four major cancer types-DS-8201!
DS8201, a HER2 antibody + irinotecan chemotherapeutic drug conjugate, belongs to the ADC type drug type (antibody conjugate drug). It consists of two parts: the first part is an antibody against the HER2 target, which can accurately identify and bind to tumor cells with high or even low expression of HER2; the second part is the highly toxic chemotherapeutic drug irinotecan. This design allows the antibody to take the chemotherapeutics to find the tumor cells, and then accurately poison the tumor cells to kill them.

01. The objective remission rate reached 60.9%, and DS-8201 strongly reversed the condition of breast cancer
The DESTINY-Breast01 clinical trial aims to evaluate the safety and efficacy of DS-8201 in HER2-positive unresectable and/or metastatic breast cancer patients who have previously received trastuzumab-metansine conjugate treatment.
For 184 patients with HER2-positive breast cancer after receiving the recommended dose of 5.4 mg/kg, the objective response rate (ORR) was 60.9%, including 11 patients whose tumors disappeared completely, the disease control rate was 97.3%, and the clinical benefit rate was 76.1 %, the median duration of response (DoR) was 14.8 months, The median progression-free survival (PFS) was 16.4 months.
The trial has not yet reached the median overall survival (OS) of patients, and the 1-year survival rate of patients is estimated to be 86%.
02. Disease control rate reached 83%, DS8201 broke the shackles of HER2-positive colorectal cancer
Colorectal cancer is the third most common cancer in the world and the second most common fatal cancer. For patients with metastatic colorectal cancer who have progressed after first-line treatment, the follow-up treatment options are limited, the patient suffers from the disease, and the prognosis is extremely poor.
DESTINY-CRC01, announced at the ASCO meeting in 2020, is a phase II clinical trial for HER2-positive, unresectable and/or metastatic colorectal cancer patients who have previously received two or more standard treatments.
The results of this test showed that after DS-8201 monotherapy, the patient’s objective tumor response rate was 45.3%, of which the complete response rate was 1.9%, the partial response rate was 43.4%, and the disease control rate (DCR) reached 83.0%. The median progression-free survival (PFS) was 6.9 months.
03. The objective remission rate reached 61.9%, DS-8201 led the lung cancer targeted therapy and set off an innovative revolution
The DESTINY-Lung01 study released at the ASCO conference this year is aimed at HER2 mutant (HER2m) unresectable and/or metastatic non-squamous non-small cell lung cancer (NSCLC) that has often received one or more systemic treatments and has disease progression Most of the patients had received platinum-containing chemotherapy (90.5%) and anti-PD-1 or PD-L1 therapy (54.8%).
The results of the study showed that 61.9% of patients who received DS-8201 monotherapy (6.4mg/kg) achieved tumor remission, and the disease control rate (90.5%) and median progression-free survival (14.0 months) were significantly improved .
Recently, DS-8201 was approved by the US FDA as a breakthrough disease therapy for the treatment of HER2 mutant metastatic non-small cell lung cancer, further proving that HER2 mutation is the target of clinical treatment and has become a new hope for patients with HER2 mutant NSCLC.
04. 51% effective! DS-8201 has become a dark horse for the treatment of advanced gastric cancer
In the clinical study of DESTINY-Gastric01, the effectiveness of DS-8201 in patients with HER2-positive advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma was evaluated.
Included 187 patients with HER2-positive gastric or gastroesophageal junction adenocarcinoma who had received at least two treatments. The objective response rate of DS-8201 patients was 51.3% (61/119), including 11 cases of complete remission (CR) and 50 Patients with partial remission (PR); disease control rate was 85.7%, median duration of remission was 11.3 months; median overall survival was 12.5 months; median progression-free survival was 5.6 months. Compared with chemotherapy, the risk of death was reduced by 41% (HR=0.59).
In view of this, the US FDA granted Enhertu breakthrough therapy designation for the treatment of HER2-positive unresectable or metastatic gastric cancer patients; and granted orphan drug designation for the treatment of gastric cancer patients including gastroesophageal junction cancer.
At the ASCO meeting, DS-8201 showed absolute strength in the treatment of gastric cancer, prostate cancer, colorectal cancer and other treatments, and it is expected to become the new standard therapy for patients with HER2-positive cancer. Currently, clinical trials for HER2-driven breast cancer, gastric cancer, colorectal cancer, lung cancer and other cancer types are underway.
However, what needs to be reminded here is that how to detect HER2 is the key to accurate cancer treatment!
Therefore, all cancer friends must choose the testing institution carefully. For example, patients who have been tested can contact Cancer Free Home (400-626-9916) for interpretation and evaluation of available treatment options.
Reduced the risk of death by 59%, the first ADC drug targeting TROP-2
IMMU-132 is here!
On April 22, 2020, the US FDA has accelerated the approval of its antibody conjugate drug Trodelvy (sacituzumab govitecan-hziy) for the treatment of adult patients with metastatic triple negative breast cancer (mTNBC) who have previously received at least 2 therapies. The approval time is one and a half months ahead of the scheduled approval date on June 2!
This means that Trodelvy is the first antibody-conjugated drug approved by the FDA for the treatment of triple-negative breast cancer and the world’s first approved antibody-conjugated drug targeting human trophoblast cell surface antigen 2 (Trop-2).
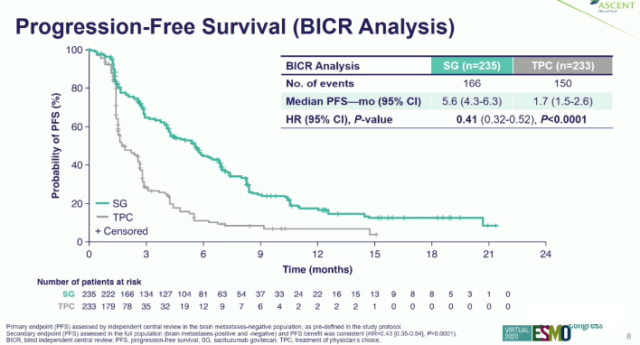
The 2020 ESMO conference announced IMMU-132 ASCENT Phase III trial data: Compared with patients with metastatic triple-negative breast cancer (TNBC) who previously chose single-agent chemotherapy, choosing sacituzumab govitecan (Trodelvy) treatment can reduce the risk of disease progression or death Reduced by 59%.
The results show:
Median progression-free survival (PFS)
The sacituzumab govitecan group was 5.6 months, and the standard treatment group was 1.7 months;
Median overall survival (OS)
It also almost doubled for 12.1 months in the sacituzumab govitecan group and 6.7 months in the standard treatment group.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



