FDA approved the first oral drug Paxlovid for COVID-19
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA approved the first oral drug Paxlovid for COVID-19
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA approved the first oral drug Paxlovid for COVID-19.
On December 22, the anti-coronavirus drug Paxlovid received an emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA).
It is used to treat mild to moderate COVID-19 pneumonia in adults and children (over 12 years old, weighing at least 40 kg or about 88 pounds) (the new coronavirus test result is positive, and there is a high risk of developing severe COVID-19 pneumonia, including hospitalization or death ).
This is also the second oral drug approved for the COVID-19 worldwide.
The COVID-19 pandemic has greatly changed our lives in an irreversible situation. Perhaps everyone has asked this sentence: When will such days end?
However, as South Africa first reported the emergence of a new mutant strain, Omicron, on November 24, the epidemic situation once again became unpredictable.
How much will the effectiveness of the vaccine be affected? What about anti-coronavirus drugs?
The good news is that on the eve of approval, Pfizer released a new press release[1] that they confirmed in in vitro experiments that Paxlovid is very concerned about mutants including Delta and Omicron. The mutant strain (VOC) still has an inhibitory effect.
As early as November, Pfizer disclosed the interim analysis results of Paxlovid’s Phase 2/3 EPIC-HR trial in its first press release [2].
In patients with mild to moderate symptoms and a higher risk of progression to severe disease Compared with placebo, receiving Paxlovid treatment within 3 days after the onset of symptoms is related to an 89% reduction in the risk of COVID-19-related hospitalization or all-cause death , which is the primary endpoint of the trial.
In addition, only 0.8% (3/389) of patients treated with Paxlovid were hospitalized for 28 days, and there was no death, while 7.0% (27/385) who received placebo had 7 deaths.
These results are statistically significant (p<0.0001).
Among patients who received treatment within 5 days of the onset of symptoms, researchers also observed a similar decrease in the risk of COVID-19-related hospitalization or all-cause death.
Among these patients, only 1.0% (6/607) who received Paxlovid treatment were hospitalized for 28 days, and there was no death, and 6.7% (41/612) who received placebo and 10 deaths.
These results are also statistically significant (p<0.0001).
In the safety assessment, the incidence of adverse events during the treatment of Paxlovid and placebo was similar (19% vs. 21%), most of which were mild. Those who were determined to be treatment-related adverse events were less severe in the Paxlovid group (1.7% vs. 6.6%) and treatment-related adverse events (2.1% vs. 4.1%) were discontinued due to treatment-related adverse events .
The press release revealed that based on the “overwhelming” efficacy advantage, Pfizer negotiated with the FDA on the recommendation of the independent data monitoring committee that the study will be terminated early.
On November 16, Pfizer announced that they had submitted an emergency use authorization application to the FDA.
The new press release [1] revealed that in the final analysis of the EPIC-HR trial , the primary endpoint and safety data of the trial were consistent with the results of the interim analysis previously disclosed.
In the secondary endpoint, the risk of all-cause hospitalization or death in patients treated within 5 days after the onset of symptoms was reduced by 88%, which was higher than the 85% in the interim analysis.
Another 2/3 stage EPIC-SR trial included hospitalization / mortality risk is low and unvaccinated , and have at least one risk factor for the progression of severe and inoculated with the vaccine to be in the patient.
Compared with placebo, the hospitalization rate in the Paxlovid group was reduced by 70%, there were no deaths, and the viral load was reduced by about 10 times.
Prior to this, another oral drug, Molnupiravir, has been approved by the British Medicines and Healthcare Products Regulatory Agency (MHRA) for the treatment of patients with mild to moderate COVID-19 pneumonia.
With the help of these drugs, the severity of the disease, the hospitalization rate and the number of deaths will be significantly reduced.
However, after Molnupiravir’s approval was announced, the mechanism that caused random mutations and induced the new coronavirus to crash during the replication process caused some safety concerns.
In contrast, Paxlovid does not seem to have this problem.
Paxlovid is a combination of protease inhibitor PF-07321332 and ritonavir. The genome of the new coronavirus encodes two polyproteins and four structural proteins.
The short non-structural proteins produced after the polyprotein is cleaved by the main protease are essential for the replication of the new coronavirus . PF-07321332 targets this The main protease M pro .
Because there is no substrate suitable for cleavage by M pro in the human body , PF-07321332 theoretically has specific selectivity for the new coronavirus [3].
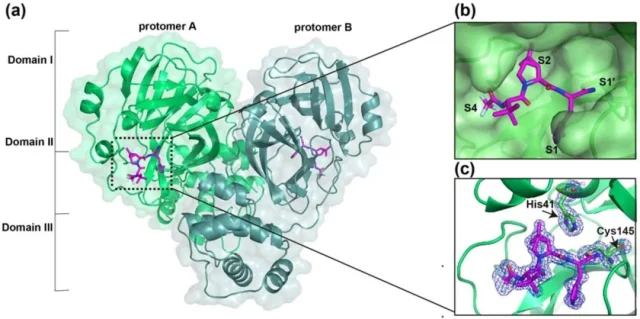
Schematic diagram of the combination of PF-07321332 and M pro
Ritonavir can inhibit the cytochrome P450 isoenzyme CYP3A, which can slow down the drug metabolism rate mediated by such enzymes.
Therefore, when combined with PF-07321332, it helps to slow down its metabolic decomposition, allowing high drug concentration The patient stays in the body for longer and better fights the virus.
In Syrian hamsters infected with the beta (B.1.351) and delta (B.1.617.2) variants of the new coronavirus, Paxlovid’s strong antiviral activity was also observed.
The lung pathology score showed that they were comparable to uninfected hamsters. The scores are almost the same [4].
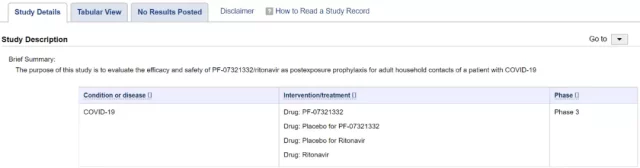
It is also worth mentioning that Paxlovid also has a clinical trial for post-exposure prophylaxis in progress [5].
The target group is family members of symptomatic patients with COVID-19 infections to block the spread of the virus within the family.
If it is effective, it can further reduce the number of patients with COVID-19 infections.
In a report in the authoritative magazine “Nature”[6], commentators pointed out that although a bright future has been seen, there are five key issues that need to be focused on.
The first is the real-world effectiveness of the drug.
The current curative effect results are only from a limited number of patients in clinical trials, the disclosed data is not complete, and the complete time window for receiving treatment has not yet been determined.
These need follow-up changes. More trials and follow-ups.
Similar key factors also include the effectiveness of mutant strains, the development of viral resistance, and the safety of drugs.
Although Paxlovid is not like Molnupiravir, it causes random mutations, but because it contains ritonavir, it is different from some commonly used ones.
Treatment of cardiovascular diseases, immunosuppressants, and analgesics may all have drug interactions that make it difficult for patients with underlying diseases to tolerate them.
In clinical applications, more monitoring and medication timing considerations are needed.
Finally, of course, it is the availability of drugs. Although Pfizer has signed an agreement to implement tiered pricing, so that low- and middle-income countries can spend less money to purchase drugs, but whether the supply of drugs can be balanced and other issues It is still a big test.
If these questions can have good answers, commentators believe that these drugs will “change the course of the COVID-19 pandemic.”
Reference:
[1] https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results
[2] https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate
[3] Owen D R, Allerton C M N, Anderson A S, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19[J]. Science, 2021: eabl4784.
[4] Abdelnabi R, Foo C S, Jochmans D, et al. The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern[J]. 2021.
[5] https://www.clinicaltrials.gov/ct2/show/NCT05047601
[6] https://www.nature.com/articles/d41586-021-03074-5
FDA approved the first oral drug Paxlovid for COVID-19
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



