A Patient with advanced cancer took three drugs and all lesions disappeared within 5 years
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
A Patient with advanced cancer took three drugs and all lesions disappeared within 5 years
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
A Patient with advanced cancer took three drugs and all lesions disappeared within 5 years.
BRAF is an important component of the RAS-RAF-MAPK signaling pathway. About 8% to 12% of patients with metastatic colorectal cancer have BRAF mutations, among which V600E is the most common mutation site.
A large number of studies have shown that BRAF V600E mutations usually occur in right-sided colon cancers, which are poorly differentiated, mucinous tumors, and often have distant metastases.
Although monotherapy with BRAF inhibitors (e.g. cannefenib, dabrafenib, vemurafenib) or MEK inhibitors (e.g. trametinib, bemetinib) in melanoma patients has a response rate of up to 60%. % to 80%, but only a 5% response rate in colorectal cancer.
In May this year, Professor Gudrun Piringer from Wels-Grieskirchen Hospital in Austria and his research team published a paper entitled “Ongoing complete response after treatment cessation with dabrafenib, trametinib, and cetuximab as third-line Treatment in a patient with advanced BRAFV600E mutated, microsatellite-stable colon cancer:
A case report and literature review” shared a case of a patient with BRAF V600E mutation and MSS colon cancer who received dabrafenib + trametinib + west A classic case of sustained complete remission after third-line treatment with tuximab.

With the combination of three drugs, the 52-year-old has regained her life
Basic information:
The patient is a 52-year-old female who was previously in good health. She visited the doctor in February 2017 because of “constipation, abdominal pain, and nausea for 3 days”.
Physical examination showed abdominal distension and mild tenderness on the left side. Blood tests showed anemia. Abdominal CT showed a 3 cm suspicious lesion in the liver and a suspicious mass in the descending colon.
Colonoscopy showed a 5 cm non-obstructive tumor in the descending colon. The biopsy confirmed that it was Colon adenocarcinoma. Liver MRI showed 2 resectable metastases in liver segments VI and VII.
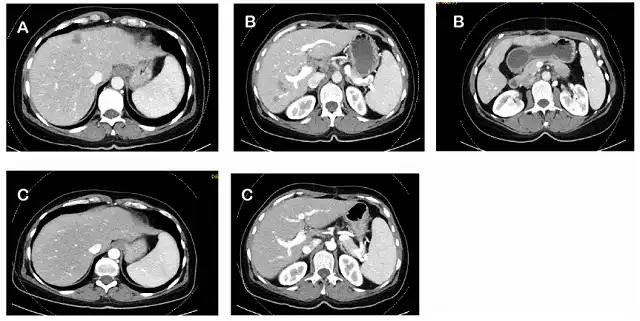
Figure 1. CT changes during treatment: (A) baseline CT in February 2017; (B) CT after CAPOX adjuvant chemotherapy in June 2017; (C) dabrafenib + trametinib in October 2018 +CT after 1 year of cetuximab
After treatment:
- In February 2017, the patient underwent subtotal colectomy with simultaneous resection of liver metastases. Postoperative pathology showed that the primary tumor was a poorly differentiated, MSS, Her2-negative, BRAF V600E mutant colon adenocarcinoma with invasion of peripheral lymphatic vessels and nerves. , positive lymph nodes (8/14); all liver metastases were resected, pathologically confirmed as metastases. FoundationOne® comprehensive genome sequencing analysis of the primary tumor showed BRAFV600E mutation, PTEN deletion, DDR1 R514C mutation, KDM5A R782Q mutation, TP53 Y234 mutation, MSS type, and the tumor mutation burden was 0 Muts/Mb;
- Adjuvant chemotherapy with capecitabine combined with oxaliplatin (CAPOX) was planned for 6 months postoperatively. However, after 3 months of adjuvant chemotherapy with CAPOX regimen, CT in June 2017 showed 5 new metastatic lesions in the liver, and the tumor marker carcinoembryonic antigen (CEA) increased;
- From June to September 2017, the patient received FOLFIRI combined with bevacizumab as first-line palliative therapy. After 3 months of treatment, CT showed that the liver metastases had progressed compared with before, accompanied by a continuous increase in CEA;
- Due to unresectable liver metastases, the patient was enrolled in the BEACON clinical trial and received FOLFIRI combined with cetuximab for 2 months. In November 2017, CT showed that the liver metastases continued to progress, with new lung metastases and retroperitoneal lymph node metastases. CEA continues to rise; patient withdraws from BEACON clinical trial;
- In December 2017, because there was no drug available, the patient began to try off-label medication, that is, the three-drug combination therapy of dabrafenib + trametinib + cetuximab. After 2 months of treatment, CT showed liver, lung and peritoneum Partial remission of posterior lymph nodes; liver, lung and retroperitoneal lymph node metastasis almost completely disappeared after 2 months of continued treatment; in August 2018, CT showed stable condition; in October 2018, PET/CT showed complete remission;
- In October 2018, the patient stopped cetuximab due to grade 3 skin toxicity (pustular papules) and repeated urinary tract infections, and continued dabrafenib + trametinib treatment, which was well tolerated;
- In February 2022, at the request of the patient, the treatment of dabrafenib + trametinib was stopped, followed by an observation and waiting strategy, and CT and blood CEA were reexamined every 3 months;
- As of January 2023, the patient remains in complete remission and is in good general condition.
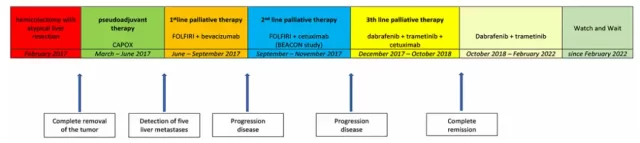
Figure 2 The whole process of patient treatment
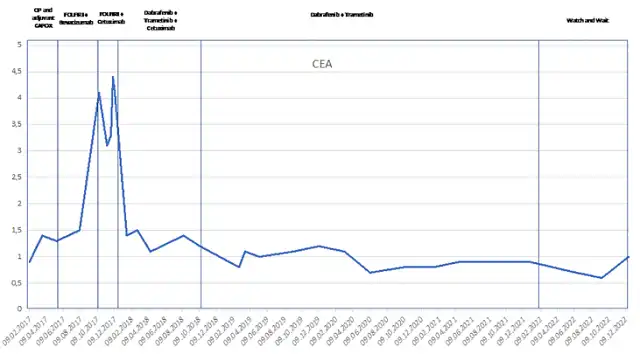
Figure 3. Changes in the tumor marker CEA during the treatment of patients from February 2017 to now
This case report is unique in the efficacy of discontinuing dabrafenib + trametinib + intermittent cetuximab as third-line therapy in patients with advanced BRAF V600E-mutated MSS-type colon cancer, even after discontinuation Sustained complete remission can also be achieved afterwards.
Dabrafenib + Trametinib + Cetuximab: What is the basis for the combination of three drugs?
First, dabrafenib used in this case is an inhibitor targeting BRAF V600E, trametinib is an inhibitor targeting MEK kinase, and cetuximab is a monoclonal antibody targeting EGFR protein .
The BRAF gene is an important member of the EGFR-RAS-RAF-MEK-ERK signaling pathway.
Although BRAF V600E mutations hardly overlap with RAS mutations in colorectal cancer, studies have found that there is a strong adaptive feedback signal in colorectal cancer, even if BRAF or MEK is inhibited, the MAPK signaling pathway can still be activated by EGFR. is activated again.
Based on this, the BRAF/MEK/EGFR three-target combination program has received solid theoretical support.
In a phase I study, 142 patients with BRAF V600E mutant colorectal cancer were randomized to receive BRAF/MEK/EGFR three-target combination therapy. The results showed that:
- Group A (dabrafenib + trametinib + panitumumab): The treatment response rate was 21%, of which 1 patient achieved complete remission, with a median PFS of 4.2 months and a median OS of 9.1 months;
- Group B (dabrafenib + panitumumab): The treatment response rate was 10%, 1 patient achieved complete remission, the median PFS was 3.5 months, and the median OS was 13.2 months;
- Group C (trametinib + panitumumab): The treatment response rate was 0%, no effective remission was reported, the median PFS was 2.6 months, and the median OS was 8.2 months.
The study further analyzed the microsatellite status of all patients and found that there was a statistically significant trend towards increased PFS in MSI patients compared with MSS patients.
None of the MSS patients benefited from the above combination therapy for more than 1 year, however one patient in the MSI cohort achieved a partial response lasting more than 24 months and another patient achieved a complete response for more than 26 months.
When BRAF V600E meets MSS
Patients with BRAF mutations usually have a high mutation burden, MSI, and CpG island methylation phenotype (CIMP). Several studies have shown that the incidence of BRAF V600E mutation in MSI patients is 34.6%, while the incidence in MSS patients is only 6.8%.
The Keynote-177 study showed that pembrolizumab was superior to chemotherapy in terms of PFS and OS, both in the overall MSI-H population and in patients with BRAF V600E mutation and MSI-H.
Even in second-line and third-line treatment, pembrolizumab showed good efficacy in patients with BRAF V600E mutation and MSI-H, with ORRs of 20% and 55%, respectively.
In addition, the CheckMate-142 study also showed that in MSI-H patients who had previously received chemotherapy, regardless of BRAF mutation, the ORR of nivolumab combined with ipilimumab was 55%, and the 12-month OS rate was 2. 85%.
However, immune checkpoint inhibitors are nearly ineffective in MSS-type colorectal cancer. Early studies showed that in patients with BRAF V600E mutation and MSS colorectal cancer, the ORR was 50% and the DCR was 96% in 22 patients treated with canafenib + cetuximab + nivolumab, but there was no The patient achieved complete remission.
In summary
The patient was unable to use immunotherapy, so he switched to the BRAF/MEK/EGFR three-target combination regimen, which achieved very significant curative effect.
After stopping the drug, he has continued to achieve complete remission, and the OS has exceeded 5 years.
The success of this case provides important guidance for exploring the treatment of metastatic colorectal cancer with BRAF V600 mutation, and we also expect that more treatment options will eventually be approved for patients with advanced colorectal cancer.
references:
Piringer G, Decker J, Trommet V, Kühr T, Heibl S, Dörfler K and Thaler J (2023) Ongoing complete response after treatment cessation with dabrafenib, trametinib, and cetuximab as third-line treatment in a patient with advanced BRAFV600E mutated, m icrosatellite -stable colon cancer: A case report and literature review. Front. Oncol. 13:1166545. doi: 10.3389/fonc.2023.1166545.
A Patient with advanced cancer took three drugs and all lesions disappeared within 5 years.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



