Olaparib + bevacizumab for advanced ovarian cancer brings durable survival benefit
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Olaparib + bevacizumab for advanced ovarian cancer brings durable survival benefit
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Olaparib + bevacizumab for advanced ovarian cancer brings durable survival benefit.
“Annals of Oncology”: The 5-year survival rate increased by 17%, and the progression-free survival rate increased by 2.5 times! Olaparib + bevacizumab first-line treatment of advanced ovarian cancer brings durable survival benefit
The phrase “turning cancer into a chronic disease” has gained increasing popularity in recent years due to advancements in precision treatment. Even in advanced cancer patients, long-term survival and even better disease-free/symptom-free survival can be achieved with effective anticancer therapy.
The treatment of metastatic/recurrent ovarian cancer serves as a good example. For ovarian cancer patients with DNA homologous recombination deficiency (HRD), such as those with BRCA1/2 gene mutations, a maintenance treatment regimen centered around PARP inhibitors significantly delays disease recurrence and improves overall prognosis.
The results of the phase III clinical study PAOLA-1, which evaluated the combination of the PARP inhibitor olaparib and bevacizumab as first-line maintenance therapy for advanced ovarian cancer, were recently published in the journal “Annals of Oncology” during the European Society for Medical Oncology (ESMO) congress.
The study showed that the 5-year survival rate for HRD-positive ovarian cancer patients treated with olaparib and bevacizumab reached 65.5%, and 46.1% of patients achieved 5-year progression-free survival (PFS).
Compared to the placebo control group receiving only bevacizumab, the addition of olaparib increased the 5-year survival rate for HRD-positive ovarian cancer patients by an absolute value of 17% and improved the 5-year PFS rate by 2.5 times (46.1%/19.2%)[1].
Such a significant increase in long-term survival rates indeed has the potential to transform this subset of ovarian cancer into a chronic disease.

Screenshot of paper home page
As one of the milestone clinical studies in the treatment of ovarian cancer with PARP inhibitors, the PAOLA-1 study included patients with advanced ovarian cancer without restricting enrollment to only those with BRCA mutations, effectively targeting the “whole population” of ovarian cancer.
The combination of olaparib and bevacizumab also significantly improved the median progression-free survival (PFS) of the overall study population [2].
However, HRD+ patients, including BRCA1 / 2 mutation-positive people, benefited most from the treatment. The median PFS of HRD+ patients in the olaparib+bevacizumab group was extended by nearly 20 months (37.2 months vs. 17.7 months).
Can the PFS benefit of the whole population of PAOLA-1 be translated into long-term OS improvement? What about the OS benefit and long-term disease-free survival in HRD+ patients? These questions are all important.
“Let time give the answer”, longer-term follow-up can answer these questions. The long-term survival results of the PAOLA-1 study officially published this time are also the final OS analysis of the research preset.
The median follow-up time of the olaparib treatment group and the control group was more than 5 years (61.7/61.9 months, OS data 55% maturity) .
Since the maximum intervention time specified in the PAOLA-1 study was 2 years, a significant proportion of patients in both groups received subsequent treatments such as PARP inhibitors, bevacizumab or chemotherapy, especially 45.7% of patients in the placebo group Subsequent use of PARP inhibitors was significantly higher than that of the olaparib group (19.7%).
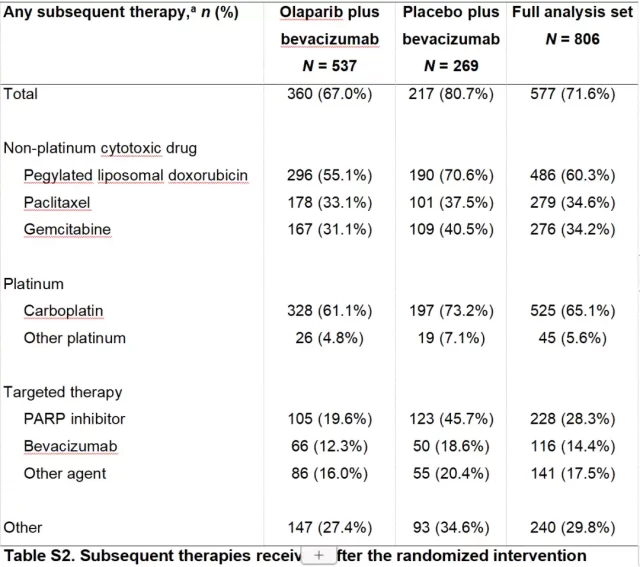
Post-study line therapy
In the final OS analysis, the researchers did not adjust the relevant data based on the impact of later-line treatment on OS.
Therefore, in the intention-to-treat population ( ITT, that is, the entire population of ovarian cancer included in the efficacy analysis) , olaparib treatment did not Compared with the placebo group, the OS was not significantly prolonged (56.5 months vs. 51.6 months, HR=0.92, P=0.4118) , and the conclusion was basically the same in the HRD-negative population (OS HR=1.19).
However, if only HRD+ patients are limited (defined as BRCA mutations in the tumor, or HRD score/genomic instability status score ≥ 42 points) , then olaparib treatment has shown a significant OS benefit, and the OS data that has not yet fully matured show that The median OS of patients in the olaparib group was nearly 18 months longer than that in the placebo control group ; the benefits of olaparib could also be observed in the BRCA mutation subgroup or HRD+non-BRCA mutation subgroup.
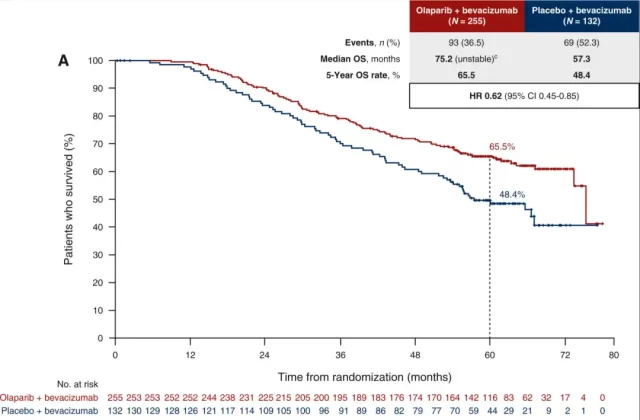
OS curve of HRD+ patients
This published paper also updated the researchers’ evaluation of PFS data of HRD+ patients: the median PFS of patients in the olaparib group was refreshed to 46.8 months, which was significantly better than that of the placebo group (HR=0.41, but not 17.6 months). Formal statistical test was carried out) , the 5-year PFS rates of the two groups were 46.1% and 19.2%, respectively .
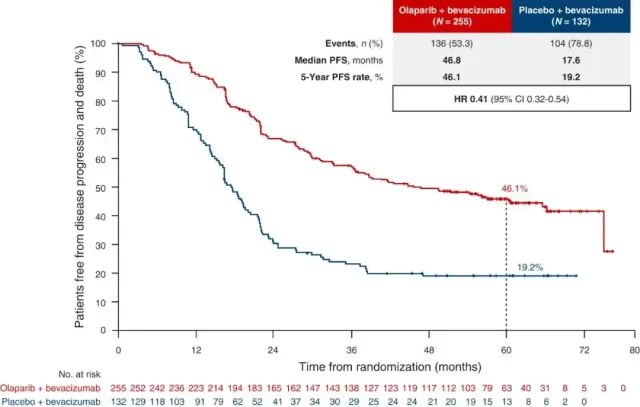
PFS curve of HRD+ patients
From the above data, it can be seen that the addition of olaparib to maintenance therapy has enabled nearly half of HRD+ patients with advanced ovarian cancer to achieve long-term disease-free survival, which is of higher quality than simple survival, and the study did not report new The safety signal is sufficient to support the widespread application of olaparib + bevacizumab as first-line maintenance therapy, and once again confirms the important value of “synthetic lethality” of PARP inhibitors.
references:
[1]Ray-Coquard I, Leary A, Pignata S, et al. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: final overall survival results from the PAOLA-1/ENGOT-ov25 trial[J]. Annals of Oncology, 2023.
[2]Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer[J]. New England Journal of Medicine, 2019, 381(25): 2416-2428.
Olaparib + bevacizumab for advanced ovarian cancer brings durable survival benefit.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



