Keytruda: The 5-year survival rate for advanced lung cancer doubled!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The 5-year survival rate for advanced lung cancer doubles! Keytruda single-agent first-line treatment long-term data
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Keytruda: The 5-year survival rate for advanced lung cancer doubled!
Recently, the ASCO journal “Journal of Clinical Oncology” published the 5-year follow-up results of KEYNOTE-024.
The latest data shows that among patients with advanced non-small cell lung cancer (NSCLC) with high PD-L1 expression, the 5-year overall survival (OS) rate of patients receiving pembrolizumab (Keytruda) is twice that of the chemotherapy group (31.9% vs 16.3%), the median overall survival time was nearly doubled (26.3 months vs 13.4 months), and pembrolizumab significantly reduced the risk of death by 38% (HR 0.62, 95% CI 0.48-0.81) .
The research team pointed out in the paper that this is the first 5-year follow-up data of a Phase 3 trial of immunotherapy for the first-line treatment of NSCLC.
These latest data confirm and strengthen the previously reported 3-year survival results, and are also consistent with the findings of the KEYNOTE-001 trial (Phase Ib).
KEYNOTE-001 determined the therapeutic benefit of Keytruda in advanced NSCLC patients with increased PD-L1 expression.
The 5-year survival rate of advanced NSCLC patients was 23.2%, and the advanced PD-L1 expression (TPS≥50%) was advanced The survival rate of newly treated NSCLC patients is approaching 30% (29.6%).

Screenshot source: Journal of Clinical Oncology
The KEYNOTE-024 trial was carried out in patients with metastatic non-small cell lung cancer (NSCLC) with PD-L1 TPS (tumor proportion score) ≥50% and no EGFR or ALK mutations, comparing pembrolizumab and platinum-containing chemotherapy as first-line The effect of treatment.
A total of 305 patients were randomized 1:1 to receive treatment. The primary endpoint of the trial is progression-free survival (PFS), and OS is the key secondary endpoint. The median follow-up time for this data analysis was as long as 59.9 months.
In the survival data of this key report, in addition to the doubling of the median overall survival and the 5-year overall survival rate, the paper specifically pointed out that although two-thirds of the chemotherapy group (99/151) patients eventually crossed the group Received immunotherapy, but pembrolizumab still showed a significant survival benefit advantage.
One of the authors of the study, Dr. Martin Reck from the Grosshansdorf Lung Center in Germany, pointed out that pembrolizumab may actually have higher therapeutic advantages over chemotherapy. “Immunotherapy drugs (including pembrolizumab) have been shown to be able to be used in the second line Treatment improves OS better than chemotherapy.
This high cross-group treatment rate may weaken the observed differences. Previous analysis showed that after adjusting for cross-group treatment factors, pembrolizumab reduced the risk of death by 51% (HR 0.49 ).”
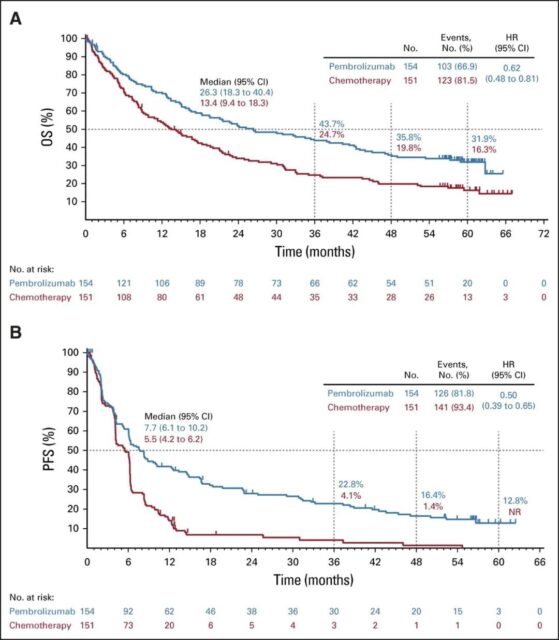
▲Survival curve (A) and progression-free survival curve (B) of pembrolizumab group and chemotherapy group
Pembrolizumab is also more advantageous in multiple other endpoints. The median PFS of pembrolizumab was 7.7 months and that of the chemotherapy group was 5.5 months (P<0.001). Pembrolizumab significantly reduced the risk of disease progression or death by 50%.
The 3-year progression-free survival rate (22.8% vs 4.1%) and the 5-year progression-free survival rate in the pembrolizumab group were higher (12.8% vs no patient evaluable).
After considering the second-line treatment, pembrolizumab still benefits significantly, the median PFS2 (progress or death after randomization to second-line treatment) is longer (24.1 months vs 8.5 months), and the 3-year PFS2 rate is higher ( 39.5% vs 15.0%).
In addition, the objective response rate assessed by the investigator in the pembrolizumab group was higher (46.1% vs 31.1%), and the median response time was longer (29.1 vs 6.3 months).
In the longer follow-up, the research team did not observe new safety signals. In each treatment group, the incidence of treatment-related adverse events (TRAE), severe TRAE, and fatal TRAE (2 cases in the pembrolizumab group and 3 cases in the chemotherapy group) was similar.
The paper concluded that in the first-line treatment of 50% of PD-L1 metastatic NSCLC, pembrolizumab provides long-lasting and clinically significant long-term survival benefits compared to chemotherapy, and has become the standard treatment for these patients .
Dr. Roy Herbst from the Yale Cancer Center commented that the impact of immunotherapy on lung cancer survival is “surprising.” Follow-up research should focus on “personalized” immunotherapy, just like personalized targeted therapy, allowing immunotherapy to be more extensive Play a role in patients, “so we can help the remaining 70% of patients who have not survived for 5 years.”
(source:internet, reference only)
Disclaimer of medicaltrend.org



