Opdivo+Yervoy for Renal cell carcinoma (RCC): 5-year survival rate is 48%
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Opdivo+Yervoy for Renal cell carcinoma (RCC): 5-year survival rate is 48%!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Opdivo+Yervoy for Renal cell carcinoma (RCC): 5-year survival rate is 48%!
First-line treatment for renal cell carcinoma (RCC)! The efficacy of Bristol-Myers Immunization Combination Opdivo+Yervoy is significantly better than Sutent (Sunitinib): the 5-year survival rate is 48%!
Up to now, the Opdivo+Yervoy combination therapy has been approved for 7 treatment indications for 6 types of cancer (melanoma, renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, non-small cell lung cancer, malignant pleural mesothelioma).
Kidney cancer (Image source: vecteezy.com)
Bristol-Myers Squibb (BMS) recently announced Opdivo at the 2020 European Society of Medical Oncology (ESMO) Virtual Conference (common name: nivolumab, Navuri) The latest results of the Phase III CheckMate-214 study (5 years of data) of the first-line treatment of advanced or metastatic renal cell carcinoma (RCC) with eumab combined with low-dose Yervoy (ipilimumab).
The results showed that Opdivo+Yervoy immunotherapy (OY combination) continued to show long-lasting long-term survival benefits: in the entire study population, nearly half (48%) of patients receiving OY combination therapy were still alive after 5 years.
The results of this update represent the longest follow-up time of any phase 3 clinical trial of the first-line RCC immune combination therapy. After a median follow-up of 67.7 months, the data continue to prove that in the primary endpoint population (patients with intermediate and high-risk [IP] prognostic factors) and the entire study population (all randomized populations, that is, the intention-to-treat population [ITT]) , OY combination maintains overall survival (OS) and remission benefits that are superior to the targeted anticancer drug Sutent (common name: sunitinib).
So far, in 6 phase 3 clinical trials of 5 tumors, the Opdivo+Yervoy combination therapy has shown a significant improvement in overall survival (OS): advanced renal cell carcinoma, non-small cell lung cancer, metastatic melanoma, malignant interpleural Dermatoma, esophageal squamous cell carcinoma.
Dr. Nick Botwood, Vice President of Oncology Development at Bristol-Myers Squibb, said: “Four years ago, we showed the first results of the CheckMate-214 trial during ESMO, which helped change the standard of care for advanced renal cell carcinoma.
Now, with the longest follow-up of any phase 3 trial of immune combination therapy in this disease, we see the true long-term survival benefits of Opdivo and Yervoy.
The results of these five-year RCC studies supplement the evidence we have seen in several other tumor types, that is, Opdivo+Yervoy-based combination therapy can play a unique role in changing the survival outcomes of cancer patients. “
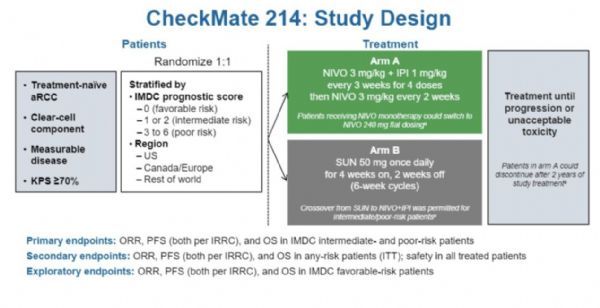 CheckMate-214 research design
CheckMate-214 research design
CheckMate-214 is a randomized, open-label study conducted in previously untreated (naïve) patients with advanced or metastatic RCC, evaluating Opdivo (3mg/kg) and low-dose Yervoy (1mg/kg) combination therapy ( O3Y1) Compared with the standard first-line therapy Sutent (sunitinib) for the efficacy and safety of the first-line treatment.
Five-year data show that compared with Sutent, OY combination therapy continues to show better overall survival (OS), objective response rate (ORR), duration of response (DOR), and complete response rate (CR).
The 67.7-month follow-up analysis in the IP patient population (n=847) showed that compared with the Sutent group, the OY group showed significant improvement in the following endpoints:
(1) In terms of OS, the median OS of the OY group was 47.0 months, and that of the Sutent group was 26.6 months (HR=0.68, 95%CI: 0.58-0.81); the 5-year survival rate of the OY group was 43%, and that of the Sutent group was 31%.
(2) In terms of ORR, the value of the OY group is higher (42% vs 27%). (
3) In terms of CR, 11% in the OY group and 2% in the Sutent group.
(4) In terms of DOR, the median DOR in the OY group has not yet been reached, and it was 19.7 months in the Sutent treatment group.
The 67.7-month follow-up analysis in the ITT patient population (n=1096) showed:
Compared with the Sutent group, patients in the OY group showed significant improvements in the following endpoints: (1) In terms of OS, the median OS was 55.7 months in the OY group and 38.4 months in the Sutent group (HR=0.72; 95%CI: 0.62- 0.85); the 5-year survival rate was 48% in the OY group and 37% in the Sutent group. (2) In terms of ORR, the value of the OY group is higher (39% vs 22%). (3) In terms of CR, 12% in the OY group and 3% in the Sutent group; a higher proportion of patients in the OY group achieved CR and there was no subsequent progression of the disease (9.6% vs 2.4%). (4) In terms of DOR, the median DOR in the OY group has not yet been reached, and it was 24.8 months in the Sutent treatment group.
Safety:
In the study, the safety profile of Opdivo+Yervoy immune combination therapy is manageable using established treatment algorithms, and there are no new safety signals during long-term follow-up.
The 5-year data from the CheckMate-214 clinical trial provided during ESMO also includes a conditional survival analysis, which estimates the probability that a patient will remain event-free (ie, remain alive, disease-free, and in remission) for a period of time after reaching the treatment milestone.
The key results include:
(1) Among patients who survived 3 years after starting the OY combination therapy, there is an 81% probability that they will continue to survive in the next 2 years, while the probability for patients who receive Sutent is 72%.
(2) Among patients who received OY combination therapy for 3 years without progression, there is an 89% probability that they will not progress within the next 2 years, while the probability of patients receiving Sutent therapy is 57%.
(3) Among patients who had remission on OY combination therapy and had remission within 3 years, there was an 89% probability that they would still be in remission within the next 2 years, while the probability of patients receiving Sutent treatment was 63%.
Renal cell carcinoma (RCC) is the most common type of kidney cancer in adults. There are more than 431,000 new cases and more than 179,000 deaths worldwide each year. The incidence of RCC in men is approximately twice that of women, with the highest incidence in North America and Europe. From historical data, the 5-year survival rate for patients diagnosed with metastatic or advanced renal cancer is only 13%.
Both Opdivo and Yervoy are tumor immunotherapy. They use the body’s own immune system to fight tumors by targeting different regulatory elements in the immune system. Opdivo targets the PD-1/PD-L1 pathway, and Yervoy targets it. CTLA-4.
In the United States and the European Union, the Opdivo+Yervoy immunotherapy combination therapy was approved in April 2018 and January 2019, respectively, becoming the first immuno-oncology combination therapy (I-O/I-O combination) for the first-line treatment of middle- and high-risk RCC patients.
Opdivo+Yervoy (OY combination) is the first and only dual immunotherapy to receive regulatory approval.
Opdivo+Yervoy is a unique combination of two immune checkpoint inhibitors, with a potential synergistic mechanism. It targets two different checkpoints (PD-1 and CTLA-4) and acts in a complementary way to help the body destroy tumors. cell.
Yervoy can help activate and proliferate T cells, while Opdivo can help existing T cells find tumors. In addition, some T cells stimulated by Yervoy also become memory T cells, which may lead to a long-term immune response.
Up to now, the Opdivo+Yervoy combination therapy has been approved for 7 treatment indications for 6 types of cancer (melanoma, renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, non-small cell lung cancer, malignant pleural mesothelioma) in different countries It is different from the region.
In addition, the Opdivo+Yervoy combination therapy has shown significant improvement in overall survival (OS) in 6 phase 3 clinical trials of 5 cancers:
- Non-small cell lung cancer (CheckMate-227, CheckMate-9LA),
- Metastatic melanoma (CheckMate-067),
- Advanced renal cell carcinoma (CheckMate-214),
- Malignant pleural mesothelioma (CheckMate-743),
- Esophageal squamous cell carcinoma (CheckMate-648).
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



