FDA approves olaparib for gBRCA-mutated metastatic pancreatic cancer
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA approves olaparib as first-line maintenance treatment for gBRCA-mutated metastatic pancreatic cancer
FDA approves olaparib as first-line maintenance treatment for gBRCA-mutated metastatic pancreatic cancer. There are other genes and mutations in the BRCA family, such as PALB2 gene, CHEK2 gene, ATM gene, etc.-they are all part of the Fanconi anemia pathway. These are mutations based on some studies in other groups. We know that these mutations are common. The question is whether we can get the same results as the BRCA mutation.
On December 30, 2019, the U.S. Food and Drug Administration (FDA) has approved the PARP inhibitor Olaparib (Olaparib) for carrying harmful or suspected harmful germline BRCA mutations (gBRCAm), at least 16 weeks on line containing platinum basis Maintenance therapy for adult patients with metastatic pancreatic cancer without disease progression during chemotherapy.
Patients with pancreatic cancer need to be tested and selected for treatment based on the FDA-approved olaparib companion diagnostic product BRAC Analysis CDx®.

Olapali is now the only approved targeted drug among advanced pancreatic cancer patients selected by biomarkers (gBRCAm). GBRCA testing in pancreatic cancer patients will be very important!
On June 2, 2019, ASCO announced the endpoints of the main phase III clinical trial of POLO.
The results were simultaneously published in “N Engl J Med” on June 2;
On July 2, 2019, the NCCN 2019.3 version of pancreatic cancer guidelines recommended olaparib as the first-line maintenance treatment for gBRCAm-mutated pancreatic cancer;
On December 30, 2019, the FDA approved olaparib as the first-line maintenance treatment for gBRCA mutant metastatic pancreatic cancer.
The US FDA Oncology Drug Advisory Committee voted 7 to 5 to approve Olaparib (Olaparib) for the first-line maintenance treatment of patients with gBRCA mutant metastatic pancreatic cancer. The FDA also approved BRACAnalysis CDx® as its companion diagnosis to determine which pancreatic cancer patients are eligible for treatment with olaparib. The purpose of BRACAnalysis CDx® is to detect germline BRCA1 and BRCA2 variants (gBRCA1/2m) and provide clinical explanations for the identified variants.
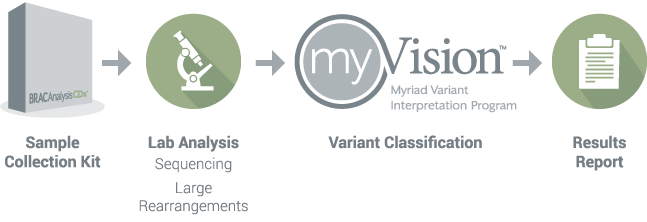
▲ BRACAnalysis CDx® detection process
This approval is based on data from a phase III POLO clinical trial, which showed that compared with placebo, olaparib’s progression-free survival (PFS) had a statistically significant and temporary improvement. Olaparib almost doubled (doubled) the PFS or death time of patients with metastatic pancreatic cancer with harmful or suspected harmful germline BRCA mutations (gBRCAm), compared with 7.4 months in the olaparib group, and The placebo control group was 3.8 months, which reduced the risk of disease progression by 47%. 2,3,5,6 In addition, after two years, 22.1% of patients in the olaparib group had no disease progression, compared with 9.6% of patients receiving placebo.
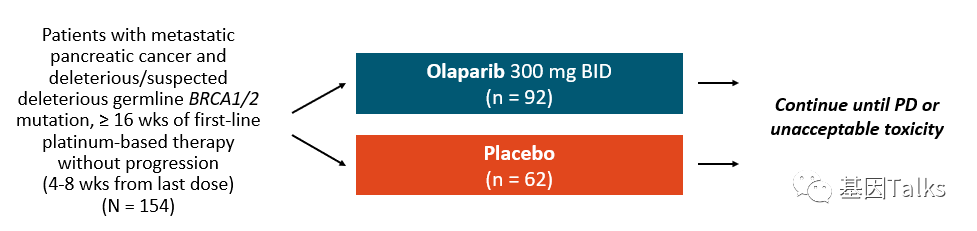
▲ POLO clinical trial program
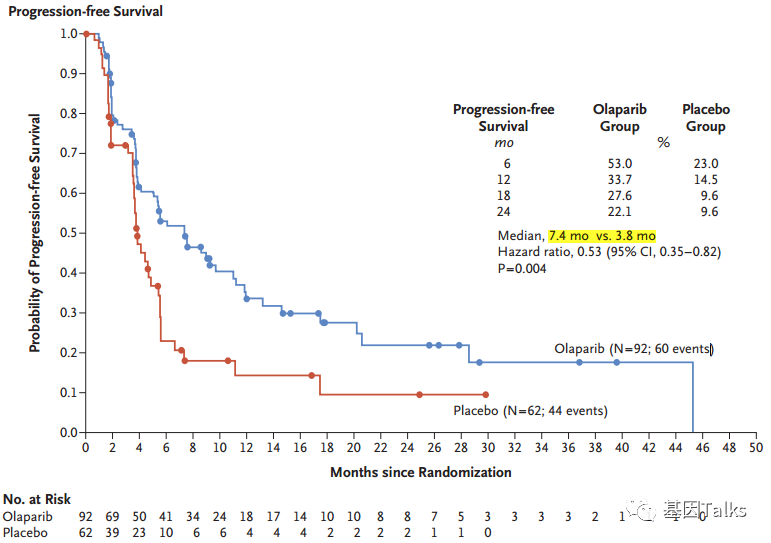
▲ POLO clinical trial olaparib vs placebo PFS
Olapali action principle
Olaparib is a veritable first-in-class in the field of PARP inhibitors, and it is also the first targeted drug that potentially uses DNA damage response (DDR) pathway defects (such as BRCA mutations) to preferentially kill tumor cells.
PARP is a vital enzyme in the process of repairing single-stranded DNA breaks. In tumor cells with harmful mutations in the BRCA gene, the BRCA1/2-mediated DNA repair pathway is defective. When the PARP-mediated DNA repair pathway is also After being inhibited, DNA breaks cannot be repaired, and DNA damage accumulates, leading to cell apoptosis, that is, Synthetic lethality. Similarly, tumor cells with BRCA gene mutations have homologous recombination repair defects (HRD), affecting the DNA double-strand break repair process, causing cancer cells to be extremely sensitive to chemotherapy drugs that induce DNA double-strand breaks.
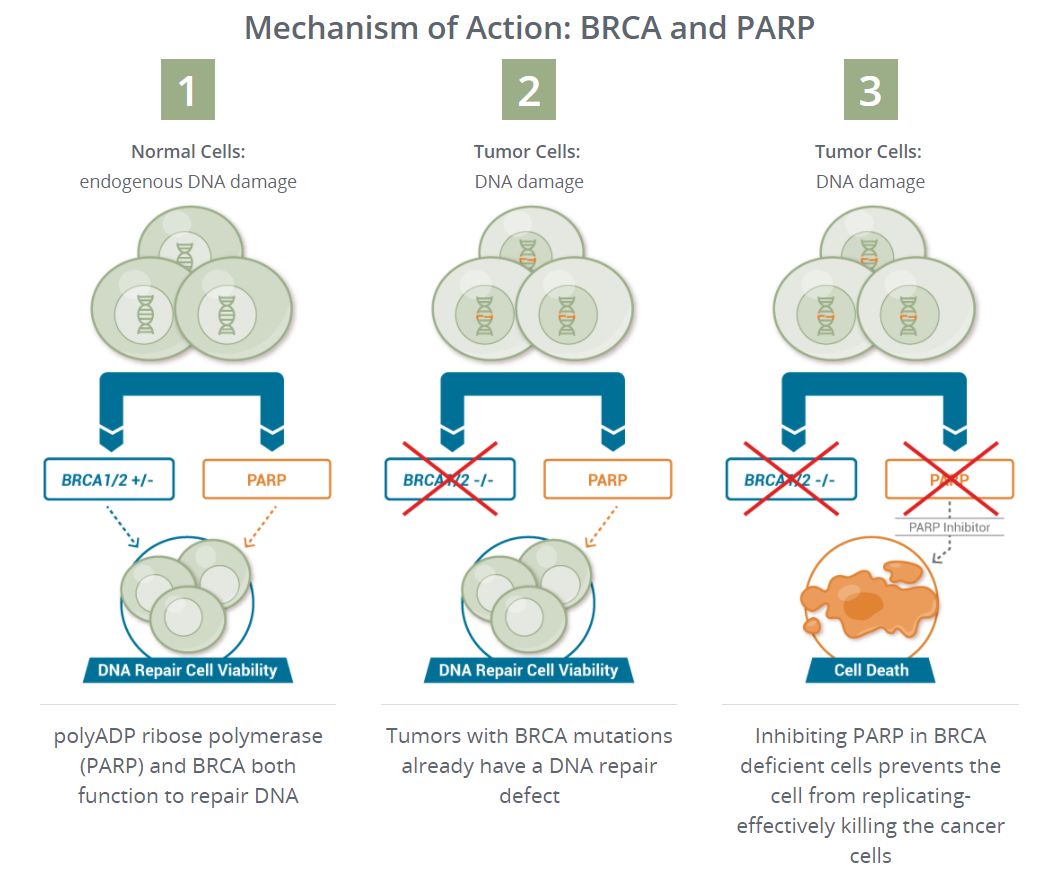
▲PARP inhibitor action principle
The future of pancreatic cancer PARP inhibitors
We know that the patients with germline BRCA (gBRCA) mutations in pancreatic cancer are about 6% to 7%, and the patients with systemic BRCA (sBRCA) mutations are about 3% to 4%. As the study shows, the FDA approval will first target germline BRCA mutations (gBRCAm), but will raise related questions so that researchers will also focus on systemic BRCA mutations (sBRCAm) in this study.
There are other genes and mutations in the BRCA family, such as PALB2 gene, CHEK2 gene, ATM gene, etc.-they are all part of the Fanconi anemia pathway. These are mutations based on some studies in other groups. We know that these mutations are common. The question is whether we can get the same results as the BRCA mutation.
There is also a large group of people called homologous recombination defective (HRD) pancreatic cancer, and about 30% of pancreatic cancers are homologous recombination defective. Can olaparib be used in this group of patients? Can olaparib be used in combination with other drugs? These are the questions that need to be explored in the future of PARP inhibitors for pancreatic cancer (previously, the PARP inhibitor niraparib has been approved by the FDA for HRD-positive advanced ovarian cancer). The answers to these questions may be seen on ASCO in 2021.
(source:internet, reference only)
Disclaimer of medicaltrend.org



