New drug for metastatic breast cancer (mBC): Halaven (Eribulin)
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New drug for metastatic breast cancer (mBC): Halaven (Eribulin)
New drug for metastatic breast cancer (mBC): Halaven (Eribulin). Eisai announced the strong efficacy data of the real-world study of Halaven (Eribulin).
Eisai recently released data from a real-world study evaluating the original anticancer drug Halaven (generic name: eribulin mesylate) in the treatment of metastatic breast cancer (mBC).

The study evaluated the treatment model and clinical results of Halaven as a third-line or multiple-line therapy for mBC patients, including triple-negative breast cancer (TNBC) subtypes. The research data was recently published in the international medical journal “Advances in Therapy” with the title of the article: Effectiveness of Eribulin in Metastatic Breast Cancer: 10 Years of Real-World Clinical Experience in the United States.
Halaven was approved by the US FDA in November 2010 for the treatment of mBC patients who have previously received at least two chemotherapy regimens for the treatment of metastatic disease. Prior treatment should include the use of anthracyclines and taxanes in the treatment of adjuvant or metastatic disease.
In China, Halaven was approved by the National Medical Products Administration (NMPA) in July 2019 for the treatment of locally recurring or metastatic breast cancer that has previously received at least 2 chemotherapy regimens (including anthracyclines and taxans). patient. In January 2020, Halaven went public in China.
This study is a retrospective, multi-site retrospective study of medical records, conducted in the practice of oncology in the United States, and included mBC patients (n=513) who started Halaven treatment based on the U.S. prescription information between 2011 and 2017. . The data is extracted by the prescribing doctor from the patient’s electronic health record and obtained through the electronic case report form. All patient data is de-identified before analysis. The clinical endpoints evaluated include the objective response rate (ORR), clinical benefit rate (CBR), progression-free survival (PFS) and overall survival (OS) reported by the provider, including all mBC patients and patients with TNBC subtypes.
The median age of these patients at the start of Havalen treatment was 59 years, and 61% of the patients had a Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1. In the entire mBC cohort, 50% (n=256) were TNBC. Compared with the overall patient cohort (78%), a larger proportion of TNBC patients received Havalen treatment in the third line (87.9%), and the remaining patients received Havalen treatment in the fourth or multiple lines. At the time of the data cutoff, 96.9% (n=497) of the entire patient cohort and 96.9% (n=248) of the TNBC subgroup had stopped Havalen treatment. Among patients who discontinued Havalen treatment, disease progression was the main reason for 78.1% of patients in the overall cohort and 84.3% of patients with TNBC subtypes, respectively.
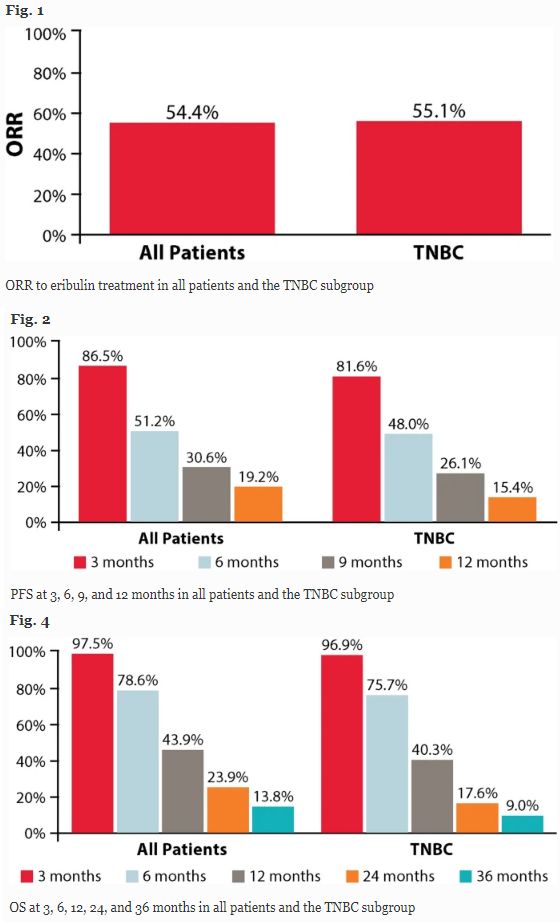
ORR-PFS-OS data
In the entire mBC cohort, the median PFS was 6.1 months (95%CI: 5.8-6.6). In the TNBC subgroup, the median PFS was 5.8 months (95%CI: 5.1-6.4). The median OS of the mBC group was 10.6 months (95%CI: 9.9-11.7), and the TNBC group was 9.8 months (95%CI: 8.6-11.0). In the entire mBC cohort, the ORR was 54.4% (95%CI: 50.1-58.7), and the TNBC subgroup was 55.1% (95%CI: 49.0-61.2). In all mBC cohorts, the CBR was 56.7% (95%CI: 52.4-61.0), and the TNBC subgroup was 57.4% (95%CI: 51.4-63.5).
One of the limitations of this study is that it did not collect detailed safety data. In addition, the treatment model reflected in the study may only represent the practice of doctors who agreed to participate in the study. If patients transfer treatment to other medical institutions and centers, they may lose follow-up during the study period.
Dr. Takashi Owa, Vice President, Chief Drug Creation Officer and Chief Discovery Officer of Eisai’s Oncology Business Group said: “For oncologists and patients with metastatic breast cancer, these data provide insights into Havalen’s real-world practices. We have always been committed to it. To provide Havalen with continuous data, whether in the real environment or in translational research related to mBC, to promote our continuous innovation in refractory diseases such as mBC.”
The active pharmaceutical ingredient of Halaven is eribulin, which is a synthetic analog of halichondrin B discovered and developed by Eisai. Halichondrin B is a natural product isolated from the sponge Halicondria okadai (okada soft sponge).
Eribulin is the first halichondrin-based microtubule kinetics inhibitor with a new mechanism of action. Eribulin is thought to work mainly through a tubulin-based mechanism that causes long-term irreversible mitotic arrest and ultimately leads to the death of apoptotic cells.
In addition, in preclinical studies of human breast cancer, eribulin showed complex effects on the tumor biology of surviving cancer cells, including increased vascular perfusion leading to reduction of tumor hypoxia, and changes in gene expression related to phenotypic changes in tumor specimens. Promote epithelial phenotype and fight against mesenchymal phenotype. Eribulin has also been shown to reduce the migration and invasiveness of human breast cancer cells.
Up to now, Halaven has been approved in many countries in Europe, America and Asia for the treatment of breast cancer. In addition, in some countries, Halaven is also approved for the treatment of soft tissue sarcoma.
(source:internet, reference only)
Disclaimer of medicaltrend.org



