Cell Metabolism: How to prevent liver damage caused by obesity?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Cell Metabolism: How to prevent liver damage caused by obesity?
Cell Metabolism: How to prevent liver damage caused by obesity? The Cincinnati Children’s Research Group reported that excessive liver fat deposition due to obesity can change the liver’s microenvironment, thereby attracting highly specific immune T cell populations to the liver. These cells help trigger NAFLD.
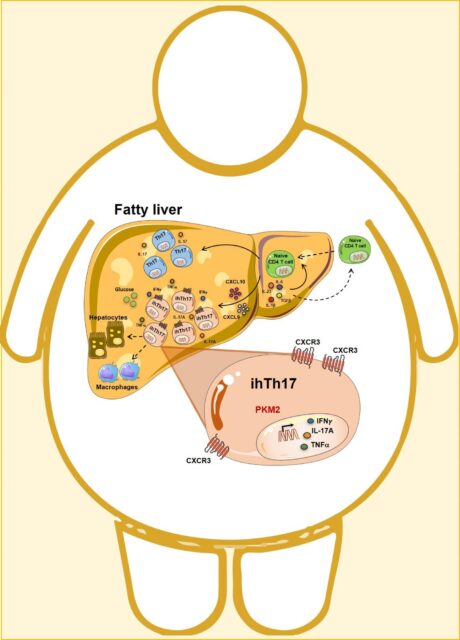
The obesity-driven fatty liver microenvironment generates a unique inflammatory hepatocyte subgroup Th17 (ihTh17) through the activation of the CXCR3 axis, which preferentially utilizes the glycolytic pathway…
Source: Cincinnati Children
When an obese person starts to accumulate fat in the liver, there is a particularly dangerous health risk of being extremely overweight.
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the world and the main underlying cause of liver transplantation in children and adults. This kind of transplantation is only suitable for a small number of patients. Without this kind of transplantation, NAFLD could be fatal over time. In fact, more than 30,000 people die from NAFLD every year (excluding alcohol-related liver damage).
For many years, the main method of treating NAFLD has been through the use of various weight control methods: diet plans, exercise programs, drugs with limited efficacy, bariatric surgery, and so on. But once people develop progressive NAFLD, simply losing weight is not enough.
Now, after years of studying the many mechanisms of obesity and NAFLD, a team of 20 scientists at the Cincinnati Children’s Center has made significant progress. Their results will be published online in the journal Cell Metabolism on May 17, 2021.
Introduction of ihTh17 cells
The research team reported that excessive liver fat deposition due to obesity can change the liver’s microenvironment, thereby attracting highly specific immune T cell populations to the liver. These “inflammatory liver CXCR3+Th17 cells” or “ihTh17” cells continue to cause excessive inflammation and life-threatening liver damage.
Through a series of experiments using human tissues and cells and multiple strains of genetically modified mice, the team found that obesity itself triggers a molecular “pathway” that starts with the overexpression of CXCL10 and CXCR3 genes. This abnormal activity attracts more and more ihTh17 cells to the liver. The result is a scorched earth inflammation feedback loop that recruits additional immune cells and gradually impairs liver function.
After tracking the liver recruitment pathway of ihTh17 cells, the team began looking for ways to break the unhealthy cycle of inflammation. They found success in mice lacking expression of the Pkm2 gene in T cells, and the Pkm2 gene seems to be the key to the continued activity of the CXCR3 pathway.
When these genetically modified mice are fed foods that induce obesity, they still get fat. But they suffered significantly less liver damage than non-transgenic mice.
Next, the researchers tested human tissue collected from NAFLD patients. They confirmed that many key genes and molecular activities that occur in mice can also be detected in human liver cells.
“Our findings are the first to prove that ihTh17 cells represent an important part of the complex world of non-alcoholic fatty liver pathogenesis,” said corresponding author Senad Divanovic, Ph.D., member of the Department of Immunobiology in Cincinnati and Maria Moreno-Fernandez. One author, Ph.D., postdoctoral fellow in the Divanovic laboratory.
Knowing more about how to regulate the function of ihTh17 cells and their interaction with liver cells and the immune system may lead to new treatments to reduce the damage caused by NAFLD.
Next step
But can the treatments used in mice also help humans? In the near future, human gene editing is unlikely to be an acceptable option for this situation. However, Divanovic said that some drugs are known to block the activity of Pkm2.
These drugs still require more in-depth laboratory evaluation. In the end, a promising compound also needs to go through many years of clinical trials. But now, for the first time in years, the team has a promising clue.
Divanovic said: “If we can regulate the unnecessary inflammatory response associated with NAFLD in a targeted way, we may be able to improve liver damage and improve the survival and health of NAFLD patients.”
(source:internet, reference only)
Disclaimer of medicaltrend.org



