‘Cancer-Shattering’ Method Targets Non-Coding Sequences to Eradicate Brain Tumors
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
CRISPR Breakthrough: ‘Cancer-Shattering’ Method Targets Non-Coding Sequences to Eradicate Brain Tumors
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
CRISPR Breakthrough: ‘Cancer-Shattering’ Method Targets Non-Coding Sequences to Eradicate Brain Tumors
Nobel Prize Team Uses CRISPR to “Shatter Cancer,” Targeting Non-Coding Sequences to Eliminate Brain Tumors.
Glioblastoma multiforme (GBM), a highly invasive cancer of the central nervous system (CNS), poses significant therapeutic challenges.
Despite employing multimodal treatment approaches, the median survival of patients is only 12-15 months.
GBM extensively infiltrates normal brain tissue, preventing complete tumor removal through surgery, leading to inevitable recurrence.
Additionally, GBM exhibits significant tumor heterogeneity, with distinct subgroups displaying various mutations, copy number variations, gene expression patterns, and epigenetic states.
This heterogeneity renders treatments targeting specific molecular processes ineffective, as tumors can recur from clones with different genomic compositions.
Temozolomide (TMZ) is the frontline chemotherapy for GBM. Sensitivity to TMZ therapy is determined by the methylation status of the O-6-methylguanine-DNA methyltransferase (MGMT) promoter.
While TMZ-based therapies show relatively fewer side effects and extend GBM patient survival, most patients still experience disease progression.
In cases of tumor recurrence, 23% of MGMT-silent GBM patients exhibit hypermutated tumors associated with poor survival.
For recurrent astrocytoma and oligodendroglioma patients, this proportion is even higher. TMZ increases the occurrence of somatic mutations, coupled with tumor genomic instability and loss of the DNA mismatch repair (MMR) pathway, resulting in hypermutation.
Crucially, there is currently no effective treatment specifically targeting hypermutated gliomas. Thus, there is an urgent need for new treatment strategies capable of eliminating GBM cells regardless of their mutations and epigenetic features.
Recently, researchers from institutions such as the Gladstone Institutes and the University of California, Berkeley, collaborated on a study published in the journal Cell Reports, titled “Targeting the non-coding genome and temozolomide signature enables CRISPR-mediated glioma oncolysis.”
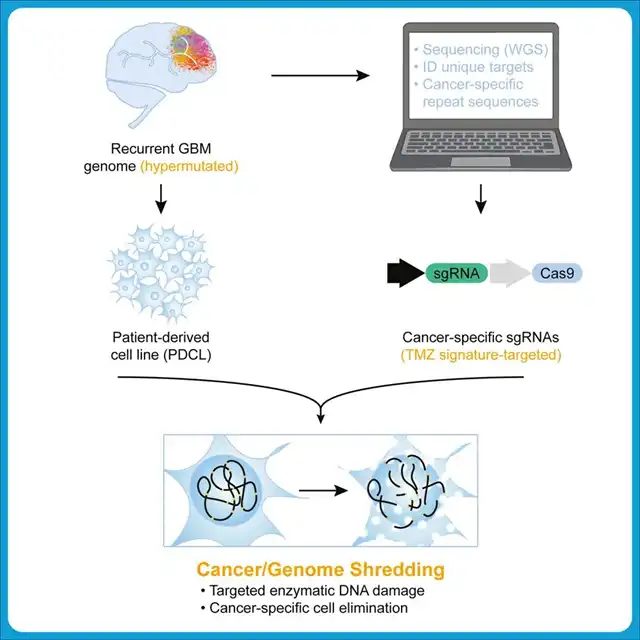
The study introduces a CRISPR-based “cancer-shattering” method that targets specific non-coding repetitive sequences to shred cancer cells, offering a potential treatment for glioblastoma.

Derived from the adaptive immune systems of bacteria and archaea, the CRISPR system induces RNA-guided DNA double-strand breaks (DSBs) at targeted genomic sites, allowing programmable gene editing in human cells. However, a potential risk of CRISPR therapy lies in the cytotoxic effects resulting from the DNA double-strand breaks it generates.
Previously, researchers like George Church and Luhan Yang used CRISPR-Cas9 gene editing to achieve 62 and 25 gene edits in pig cell lines and primary cells within living pigs, respectively, to construct gene-edited pigs for potential human organ transplants. However, it remains unclear how many such DNA double-strand breaks are needed to eliminate a cell and whether this can be exploited as an effective anti-cancer method.
In this latest study, focusing on central nervous system cancers, the research team proposes a novel strategy for treating recurrent hypermutated gliomas, termed CRISPR “cancer-shattering.” They target unique repetitive sequences in the tumor genome using the cell-toxic effects of DNA double-strand breaks generated by CRISPR-Cas9 gene editing.
Specifically, the researchers identified reproductive cell defects in DNA damage response (DDR) genes, which may lead to the high mutation load in primary GBM tumors and somatic mutations in the telomerase reverse transcriptase (TERT) promoter crucial for GBM cell immortalization. Moreover, recurrent GBM differs genetically from primary GBM, with additional mutations related to malignant tumor progression and hypermutation.
The study also found that TMZ treatment, a frontline therapy for GBM, induces hypermutation in recurrent GBM, resulting in unique, cancer-specific non-coding repetitive sequences. Importantly, these non-coding repetitive sequences can be targeted and cut by CRISPR-Cas9, causing fragmentation of the cancer cell genome at these sites, leading to cell death due to DNA damage. This selectively eliminates patient-derived recurrent GBM cell lines while preserving normal cells.
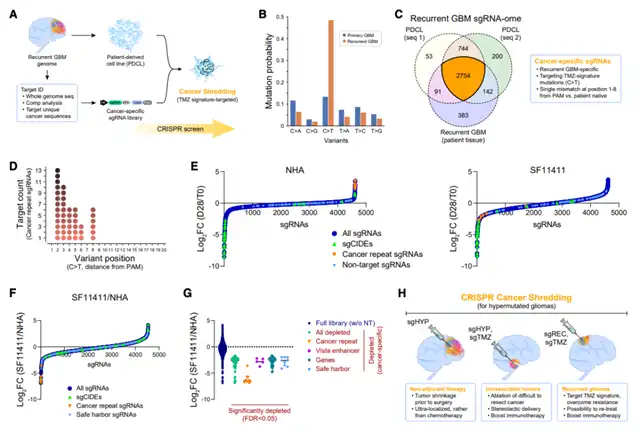
This CRISPR-based cancer-shattering approach introduces an innovative treatment paradigm independent of the tumor’s genetic and epigenetic origins, converting the tumor mutation burden and TMZ chemotherapy signal of hypermutated cancers into a potential therapeutic pathway.
Paper Link: https://doi.org/10.1016/j.celrep.2023.113339
CRISPR Breakthrough: ‘Cancer-Shattering’ Method Targets Non-Coding Sequences to Eradicate Brain Tumors
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



