Major Breakthrough: Brain tumors are finally entering the era of targeted therapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Major Breakthrough: Brain tumors are finally entering the era of targeted therapy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
NEJM Major Breakthrough: Brain tumors are finally entering the era of targeted therapy丨Clinical discovery
The treatment of low-grade glioma is finally about to change!
Recently, Professor Ingo K. Mellinghoff of Memorial Sloan Kettering Cancer Center (MSKCC) reported the exciting data of the INDIGO study at the plenary session of the American Society of Clinical Oncology (ASCO) annual meeting.
This randomized, double-blind, placebo-controlled phase 3 clinical study demonstrated that for patients with grade 2 gliomas carrying IDH mutations who had only undergone surgery, compared with placebo-treated control patients, daily A single oral dose of vorasitinib reduced patients’ risk of disease progression or death by 61% .
Specifically, the median progression-free survival (PFS) was 11.1 months in the placebo group and 27.7 months in the vorasidenib group (P<0.001).
It is especially worth mentioning that during the course of the study, the researchers noticed that vorasidenib had an amazing effect, so the study was unblinded in advance , and the patients in the placebo group also received treatment opportunities in advance.
It is understood that vorasidenib is the first new option for patients with low-grade glioma in more than 20 years, and it is also the first exclusive targeted therapy drug for glioma .
Relevant research papers were simultaneously published in the top medical journal “New England Journal of Medicine” [1].
Screenshot of paper homepage
Glioma is the most common malignant primary brain tumor in adults.
IDH gene mutations exist in almost all grade 2 gliomas , and the combination of radiotherapy and chemotherapy is the standard treatment for grade 2 gliomas [2].
Although postoperative adjuvant chemotherapy and radiotherapy can control the progression of the disease, the patients are still not cured, and these therapies have long-term toxic effects such as affecting cognition [3-5].
In order to avoid these toxicities, many patients with grade 2 gliomas carrying IDH mutations do not receive adjuvant chemoradiotherapy immediately after surgery, but instead monitor the tumor with MRI and start chemoradiotherapy when necessary[6,7].
Therefore, if a drug can be developed to control the progression of the disease after surgery and delay the use of chemotherapy and radiotherapy as much as possible, it will undoubtedly improve the quality of life of patients and even change the general treatment of glioma .
It is against this background that the IDH inhibitor vorasidenib came into being. As an orally administered small molecule compound, vorasidenib can penetrate the blood-brain barrier [8].
Early preclinical and clinical studies confirmed its safety and potential antitumor activity [9,10]. The INDIGO study led by Mellinghoff is to explore the anti-tumor effect of vorasidenib in the Phase 3 clinical study.
Between January 2020 and February 2022, a total of 331 patients with grade 2 glioma with IDH mutation who had only undergone surgery and carried IDH mutations were enrolled in the INDIGO study at 77 centers in 10 countries, of which 21.5% Patients had received two or more surgeries before enrollment.
168 patients were randomly assigned to the vorasidenib group (median age, 40.5 years) and 163 patients to the placebo group (median age, 39 years).
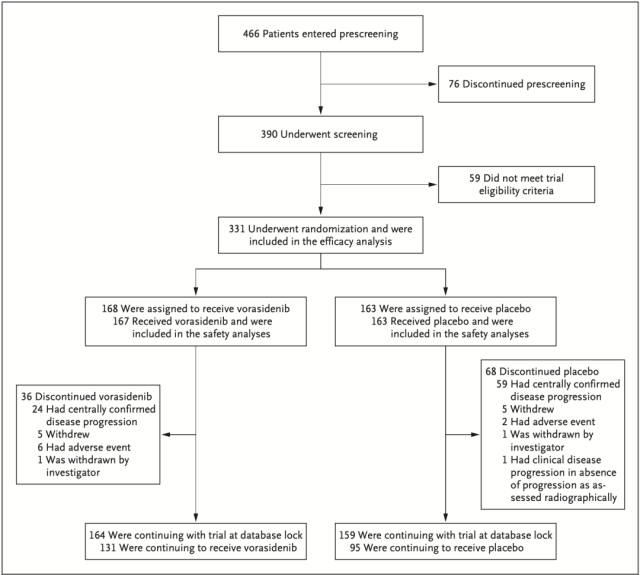
Research flow chart
During the median follow-up period of 14.2 months, no patients were lost to follow-up, and no patient deaths occurred. There were 135 patients with imaging progression events assessed by independent blind review: 47 (28.0%) in the vorasidenib group, There were 88 (54.0%) in the placebo group.
From imaging data, the median PFS was 27.7 months in the vorasidenib group and 11.1 months in the placebo group (hazard ratio for progression or death, 0.39; P<0.001).
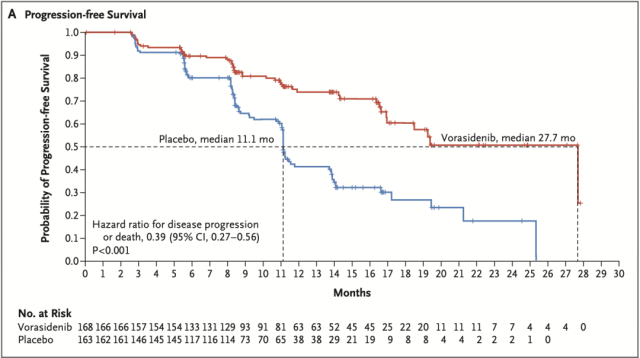
Median PFS data of the two groups
Time to other therapies was significantly improved in the vorasidenib group compared with the placebo group (hazard ratio, 0.26; P<0.001). Specifically, by 18 months, the probability of not receiving other therapies was 85.6% in the vorasidenib group and 47.4% in the placebo group; by 24 months, this probability was 83.4% and 27.0%, respectively.
A total of 77 patients received other anticancer therapies before the study was unblinded. Among them, 58 patients (35.6%) in the placebo group received vorasidenib (52 patients [31.9%]), surgery, chemotherapy, or radiotherapy; 19 patients (11.3%) in the vorasidenib group received surgery, chemotherapy, or radiotherapy.
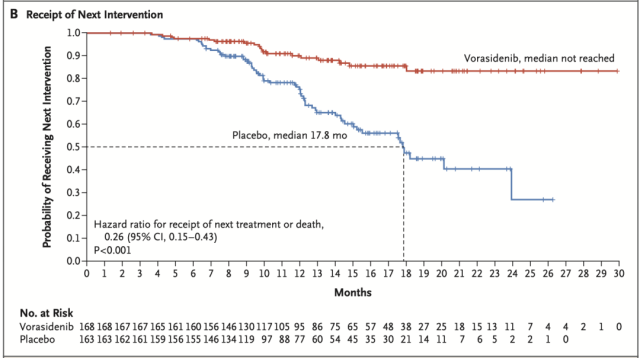
Comparison of two groups starting treatment with other therapies
In terms of safety, adverse events of grade 3 or higher occurred in 22.8% of patients receiving vorasitinib and in 13.5% of patients receiving placebo .
The most common adverse event of grade 3 or higher was increased alanine aminotransferase levels, which occurred in 9.6 percent of patients in the vorasidenib group and none in placebo-treated patients.
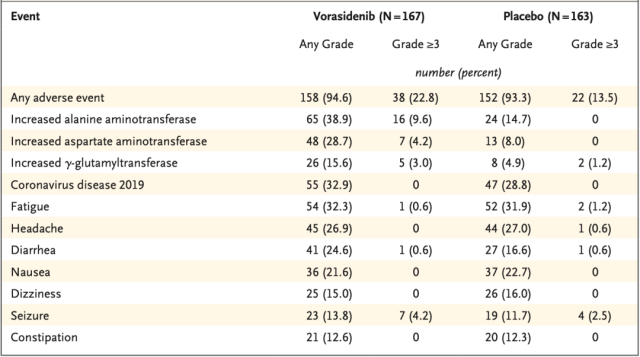
Occurrence of common adverse events
Overall, data from the INDIGO study initiated by Mellinghoff’s group showed that in patients with grade 2 gliomas harboring IDH mutations, vorasidenib significantly improved progression-free survival and delayed the start of the next anticancer therapy .
Follow-up studies investigating the effect of vorasitinib on overall survival are ongoing.
It is understood that just over two months ago, vorasidenib was granted fast-track qualification by the US FDA.
references:
[1].Mellinghoff IK, van den Bent MJ, Blumenthal DT, et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med. 2023;10.1056/NEJMoa2304194. doi:10.1056/NEJMoa2304194
[2].Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med. 2016;374(14):1344-1355. doi:10.1056/ NEJMoa1500925
[3].Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360 (9343):1361-1368. doi:10.1016/s0140-6736(02)11398-5
[4]. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810-818 .doi:10.1016/S1474-4422(09)70204-2
[5]. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189-193. doi:10.1126/science.1239947
[6].Mohile NA, Messersmith H, Gatson NT, et al. Therapy for Diffuse Astrocytic and Oligodendroglial Tumors in Adults: ASCO-SNO Guideline. J Clin Oncol. 2022;40(4):403-426. doi:10.1200/ JCO.21.02036
[7]. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170-186. doi:10.1038 /s41571-020-00447-z
[8]. Konteatis Z, Artin E, Nicolay B, et al. Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant Dual Inhibitor of Mutant IDH1 and 2 for Treatment of Glioma. ACS Med Chem Lett. 2020;11(2):101-107. doi:10.1021/acsmedchemlett.9b00509
[9].Mellinghoff IK, Penas-Prado M, Peters KB, et al. Vorasidenib, a Dual Inhibitor of Mutant IDH1/2, in Recurrent or Progressive Glioma; Results of a First-in-Human Phase I Trial. Clin Cancer Res . 2021;27(16):4491-4499. doi: 10.1158/1078-0432.CCR-21-0611
[10].Mellinghoff IK, Lu M, Wen PY, et al. Vorasidenib and ivosidenib in IDH1-mutant low-grade glioma: a randomized, perioperative phase 1 trial. Nat Med. 2023;29(3):615-622. doi:10.1038/s41591-022-02141-2
Major Breakthrough: Brain tumors are finally entering the era of targeted therapy
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



