The most toughest neurotoxicity of CAR-T cell therapy is finally solved
- Early Biomarker for Multiple Sclerosis Development Identified Years in Advance
- Aspirin Found Ineffective in Improving Recurrence Risk or Survival Rate of Breast Cancer Patients
- Child Products from Aliexpess and Temu Contain Carcinogens 3026x Over Limit
- Daiichi Sankyo/AstraZeneca’s Enhertu Shows Positive Results in Phase III DESTINY-Breast06 Clinical Trial
- Mn007 Molecules Offer Potential for Combating Streptococcus pyogenes Infection
- Popular Indian Spices Banned in Hong Kong Over Carcinogen Concerns
The most toughest neurotoxicity of CAR-T cell therapy is finally solved
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
“Nature Medicine”: The most toughest neurotoxicity of CAR-T cell therapy is finally solved!
After five years, the team of Michel Sadelain of Memorial Sloan Kettering Cancer Center finally met with you with exciting clinical research data.
On July 03, the Sadelain team published a major research result in “Nature Medicine”, announcing one of the most common and troublesome toxicity of CAR-T cell therapy, immune effector cell-associated neurotoxicity syndrome (ICANS) , found a reliable solution .
The interim data of this phase 2 clinical study initiated by them showed that the prophylactic use of IL-1 receptor antagonist anakinra can significantly reduce the incidence of ICANS caused by CAR-T cell therapy targeting CD19 without affecting CAR-T The therapeutic effect of cells.
Specifically, ICANS of any grade occurred in 22% of patients treated with axicabtagene ciloleucel (axi-cel) and brexucabtagene autoleucel (brexu-cel), and ICANS of grade ≥3 occurred in 11% of patients .
Be aware that in previous clinical trials and real-world data, axi-cel and brexu-cel reported 55-69% of any grade ICANS and 31-38% of grade ≥3 ICANS [2-5]. It is not difficult to see that these preliminary data show that anakinra did significantly reduce the incidence of ICANS.
To the best of our knowledge, this is also the first prospective clinical trial so far in which the prophylactic use of anakinra reduces the risk of ICANS associated with CD19-targeting CAR-T cell therapy .

Screenshot of paper home page
During the clinical use of CAR-T cell therapy, there are two most frightening toxicities, one is ICANS introduced earlier, and the other is cytokine release syndrome (CRS), known as “cytokine storm”.
CRS has been studied more in recent years, IL-6 receptor antagonist tocilizumab (tocilizumab) has been approved by the FDA for the treatment of CRS [6].
As for ICANS, there is currently no targeted and effective therapy , and corticosteroids are commonly used in clinical treatment [7].
Some scientists have studied the effect of prophylactic use of corticosteroids and found that the incidence of severe ICANS does tend to decrease (13%-17%), but the incidence of ICANS of any grade is still as high as 58-61% [8,9].
The breakthrough results of ICANS appeared in 2018. On May 28 of that year, “Nature Medicine” published the important research results of Sadelain’s team [10] and Attilio Bondanza’s team [11] back to back.
Both studies indicated that IL-1 released by macrophages is the key to CAR-T cell therapy leading to ICANS. Sadelain’s team also found that the preventive use of anakinra could reduce the incidence of ICANS in mice receiving CAR-T cell therapy [10].
In order to further confirm the effect of anakinra in humans, under the leadership of Sadelain’s team Jae H. Park, a single-arm, phase 2 clinical trial (NCT04148430) at Memorial Sloan Kettering Cancer Center was launched. This study included two cohorts.
Between November 11, 2019, and April 5, 2021, cohort 1 enrolled 31 patients. The median age of all patients was 62 years (range: 25-77 years), 58% were diagnosed with relapsed/refractory large B-cell lymphoma (LBCL), and 19% were diagnosed with high-grade B-cell lymphoma (HGBCL) ), 13% were diagnosed as mantle cell lymphoma (MCL), and the remaining 10% were primary mediastinal B-cell lymphoma (PMBCL). The median previous treatment of all patients was third-line (range: 2-5).
In cohort 1, 23 patients received axi-cel, 4 patients received brexu-cel, and 4 patients received tisagenlecleucel (tisa-cel). All patients received anakinra subcutaneously at a dose of 100 mg every 12 hours for at least 10 days .
The primary endpoint of the study was the incidence of severe (grade ≥3) ICANS within the first 28 days after CAR-T cell infusion.

Research design
Overall, 25 patients (81%) started anakinra on day 2 after CAR-T cell infusion and 6 (19%) started before day 2, with a median duration of anakinra administration of 11 days (range: 10-27 days). All patients tolerated treatment and completed a minimum of 10 days of anakinra treatment as per protocol, and no patient discontinued treatment due to any adverse event .
During the study period, a total of 6 patients (19%) developed ICANS of any grade; 3 patients (9.7%) had grade 1 and another 3 patients (9.7%) had grade 3 ICANS . No Level 2, 4 or 5 ICANS occurred.
Specifically, any grade and severe ICANS occurred in 22% (6 patients) and 11% (3 patients) of the 27 patients treated with axi-cel and brexu-cel; 4 received tisa-cel of patients did not develop ICANS of any grade. After CAR-T cell infusion, ICANS occurred with a median time of 4.5 days and a median duration of 4 days.

Occurrence of CRS and ICANS at different levels, and ICANS occurrence of different therapies
Of note, clinical studies and real-world data studies of axi-cel and brexu-cel have reported 55-69% of any grade ICANS and 31-38% of ≥3 grade ICANS for axi-cel and brexu-cel [ 2-5].
It is not difficult to find that the prophylactic use of anakinra not only reduces the incidence of severe ICANS, but also reduces the incidence of any level of ICANS.
Even compared with the corticosteroids mentioned above, the preliminary data of anakinra have clear advantages.
So will the preventive use of anakinra reduce the therapeutic effect of CAR-T cells? Preliminary research data suggest that this concern may be unfounded.
In the study with a median follow-up of 16 months (range: 1-24 months), 24 of 31 patients had a best overall response (77%) in partial response or better by Day 100 .
Twenty patients achieved a complete response (65%) and four patients achieved a partial response (13%).
In terms of tumor types, the ORRs of DLBCL, HGBCL, and PMBCL were 78%, 67%, and 67%, respectively; the ORRs of axi-cel, tisa-cel, and brexu-cel were 78%, 50%, and 100%, respectively.
According to the Kaplan-Meier estimate, the sustained remission rate of at least 3 months was 83%. The median progression-free survival (PFS) was 16 months. Overall survival (OS) at 1 year was 68%; median OS was not reached.

Effect of Anakinra on curative effect
The Sadelain team believes that the above data show that the preventive use of anakinra does not seem to affect the efficacy of CAR-T cells .
Because in this study, the complete remission rate of patients treated with axi-cel was 61%, the 1-year PFS rate was estimated to be 52%, which is similar to the complete remission rate reported in the previous axi-cel study with a similar median follow-up time (54 -64%) and 1-year PFS rate (44-45%) are comparable [3,12].
It is worth mentioning that Sadelain’s team also explored the impact of preventive use of anakinra on ICANS from the perspective of biomarkers.
They found that most of the cytokines that had been changed before CAR-T cell therapy did not change significantly after using anakinra. This further confirms the therapeutic effect of anakinra.
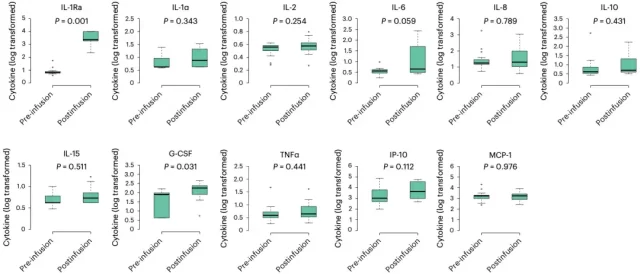
Cytokine changes
In summary, this phase 2 study initiated by Jae H. Park et al. showed that the prophylactic use of anakinra has good activity in reducing the incidence of ICANS in patients receiving CD19-targeted CAR-T cell therapy, which provides a basis for subsequent clinical exploration. Provides strong support.
It is hoped that anakinra will achieve greater success in follow-up studies and help patients receiving CAR-T cell therapy get rid of the problem of ICANS.
references:
[1].Park, JH, Nath, K., Devlin, SM et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat Med, 2023. https://doi .org/10.1038/s41591-023-02404-6
[2].Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382(14):1331-1342. doi :10.1056/NEJMoa1914347
[3].Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38( 27):3119-3128. doi:10.1200/JCO.19.02104
[4].Pennisi M, Jain T, Santomasso BD, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020;4(4):676-686. doi:10.1182/bloodadvances.2019000952
[5]. Riedell PA, Hwang WT, Nastoupil LJ, et al. Patterns of Use, Outcomes, and Resource Utilization among Recipients of Commercial Axicabtagene Ciloleucel and Tisagenlecleucel for Relapsed/Refractory Aggressive B Cell Lymphomas. Transplant Cell Ther. 20 22;28( 10):669-676. doi:10.1016/j.jtct.2022.07.011
[6]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-b-cell-all-and-tocilizumab-cytokine-release-syndrome
[7]. Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22(2):85-96. doi:10.1038/s41577-021-0054 7 -6
[8].Oluwole OO, Bouabdallah K, Muñoz J, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. 2021;194(4):690-700. doi:10.1111/ bjh.17527
[9].Topp MS, van Meerten T, Houot R, et al. Earlier corticosteroid use for adverse event management in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. 2021;195(3):388-398 .doi:10.1111/bjh.17673
[10].Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24 (6):731-738. doi:10.1038/s41591-018-0041-7
[11].Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6 ):739-748. doi:10.1038/s41591-018-0036-4
[12].Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531-2544. doi:10.1056 /NEJMoa1707447
The most toughest neurotoxicity of CAR-T cell therapy is finally solved
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.