Inducing CAR-T cells in vivo expected to break through the barriers of current cell therapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Inducing CAR-T cells in vivo expected to break through the barriers of current cell therapy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Inducing CAR-T cells in vivo expected to break through the barriers of current cell therapy.
Chimeric antigen receptor T cell therapy (CAR-T) has achieved impressive success in the treatment of hematological malignancies, but current systemic toxicity and complex manufacturing processes of autologous CAR-T cell therapy hinder its wider application .
Universal CAR-T cells have been developed to simplify the production process by isolating and editing allogeneic T cells from healthy people, but allogeneic CAR-T cells have recently encountered safety problems, and some clinical trials have been suspended by the FDA. Therefore, there is an urgent need to find new ways to overcome the current barriers of CAR-T cell therapy.
In a mouse model, in vivo CAR-T cells induced by nanocarriers loaded with CAR genes and gene editing tools showed efficacy in treating leukemia and lower systemic toxicity.
In situ programming of autologous T cells avoids the safety issues of allogeneic T cells, and the fabrication of nanocarriers is easier to standardize. Therefore, in vivo induced CAR-T cells are expected to overcome many limitations of current CAR-T cell therapy.
Recently, Researchers published a paper titled: In-Vivo Induced CAR-T Cell for the Potential Breakthrough to Overcome the Barriers of Current CAR-T Cell Therapy in Frontiers in Oncology.
CAR-T cells are expected to break through the barriers of current CAR-T cell therapy) .
This paper reviewed the application of CAR structure, gene editing tools and gene delivery technology in immunotherapy, as well as the future trend of immune cell therapy , which is helpful for the design and development of new in situ induced CAR-T cells in vivo.

Inducing CAR-T cells in vivo expected to break through the barriers of current cell therapy
Chimeric antigen receptor T cell therapy (CAR-T) is a new type of cellular immunotherapy technology, which combines synthetic receptors into T cells to recognize and kill tumor cells with cognate targeting ligands.
Since the US FDA first approved CD19-targeted CAR-T cell therapy, CAR-T cell therapy has shown unprecedented therapeutic effects in patients with B-cell lymphoma.
However, with the remarkable achievements of CAR-T cell therapy, many systemic toxic effects such as cytokine release syndrome (CRS) and neurotoxicity have also been frequently reported.
In addition, the complex manufacturing process of CAR-T cells limits the widespread application of this therapy as a standard clinical treatment.
Therefore, there is an urgent need to develop a novel CAR-T cell paradigm to overcome these barriers and allow this therapy to benefit more patients.
To simplify the complex manufacturing process of CAR-T cells, universal allogeneic CAR-T cells from healthy people have been tested in clinical trials. Universal CAR-T cells can be readily available and then infused into patients like ordinary drugs, without waiting for autologous T cells to be isolated from patients.
However, last year’s death during the clinical trial of Cellectis’ generic CAR-T therapy UCARTCS1A raised concerns about the safety of allogeneic CAR-T cells.
The FDA also recently halted clinical trials of universal CAR-T cells from Allogene due to safety concerns. Therefore, new strategies are needed to overcome the associated toxicity and simplify the manufacturing process of current CAR-T cell therapies.
In vivo CAR-T cells induced by nanocarriers loaded with CAR genes and gene editing tools have shown promising effects on leukemia.
In situ programming of autologous CAR-T cells can enhance targeted killing of tumor cells and reduce systemic toxicity such as cytokine release syndrome (CRS) and neurotoxicity.
Furthermore, nanocarriers can be easily fabricated in standardized methods. Induced CAR-T cells in vivo offer a potential solution to overcome the current barriers of CAR-T cell therapy.
This article reviews the future trends in CAR structural design, gene editing tools, gene delivery systems, and immune cell therapy.
CAR structure and evolution
The structure of chimeric antigen receptors (CARs) has a modular design, including an antigen-binding domain, a hinge, a transmembrane domain, and an intracellular signaling domain (Fig. 1A) .
The antigen-binding domain is usually a monoclonal antibody-derived single-chain variable fragment (scFv) molecule that can bind to antigens on the surface of malignant cancer cells, and the transmembrane domain is responsible for anchoring the CAR to the T cell membrane.
The intracellular signaling domain usually contains a T-cell activation domain derived from the T-cell receptor CD3ζ chain, and a co-stimulatory domain, usually consisting of a tyrosine-based immunoreceptor activation motif containing a CD28 or 4-1BB region (also called CD137 and TNFRSF9) .
Variations in each component of the CAR structure enable fine-tuning of the function and antitumor activity of the final CAR-T cell product.
Various CAR constructs have been designed to improve the safety and efficacy of CAR-T cell therapy.
Once the designed CAR gene is integrated into T cells, the scFv on the surface of T cells will specifically recognize tumor-associated antigens and bind CAR-T cells to tumor cells.
After this, the intracellular signaling domain of CAR-T cells is activated, causing CAR-T cells to proliferate and secrete cytokines that kill tumor cells.
Since Carl June of the University of Pennsylvania and David Porter of the Children’s Hospital of Philadelphia first applied CAR-T cells to clinical treatment in 2011 , the CAR structure has undergone five generations of development (Fig. 1B) .
The first-generation CARs contain intracellular stimulatory domains and extracellular single-chain antibodies. Due to the lack of co-stimulatory molecules, this generation of CAR-T cells cannot continue to proliferate. The second-generation CAR adds a co-stimulatory molecule, such as CD28, or 4-1BB (CD137) to enhance the proliferation of CAR-T cells and reduce toxicity.
Yescarta™ and Kymriah™, the two earliest approved CAR-T cell therapies, are second-generation CAR-T cells containing CD28 and 4-1BB respectively.
Third-generation CARs include two co-stimulatory molecules, such as CD27, CD28, tumor necrosis factor superfamily 4 (OX40, also known as CD134) , 4-1BB (CD137) , or CD244. The fourth-generation CAR is called TRUCKs, which combines the direct anti-tumor ability of CAR-T cells and the immunomodulatory function of delivered cytokines.
TRUCKs have entered early clinical trials using a range of cytokines, including IL-7, IL-12, IL-15, IL-18, IL-23, and combinations thereof. The fifth generation integrates an additional membrane receptor to control the activation of CAR-T cells in an antigen-dependent manner.
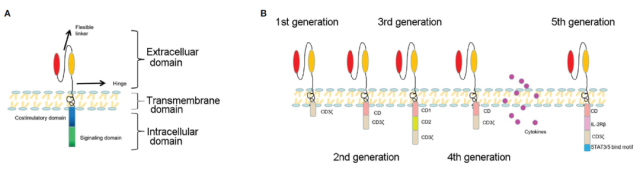
Figure 1A: Basic structure of CAR; Figure 1B: Development of five generations of CAR
In addition to adding new functional molecules in the CAR structure, many studies have selected alternative tumor targeting sites for the new CAR structure.
CD30 is very strongly expressed on Hodgkin’s lymphoma malignant cells and weakly expressed on healthy lymphocytes and hematopoietic stem/progenitor cells (HSPCs) .
CD30 CAR-T cell therapy showed excellent results in the treatment of CD30+ malignancies, while healthy activated lymphocytes and HSPCs were not affected.
CD20 is a 33-37-kDa non-glycosylated transmembrane phosphorylated protein that contributes to the development and differentiation of B cells, CD20 is highly expressed in late pre-B cells and mature B cells, but is expressed on the surface of heat-shocked cells not express.
CD20 CAR-T cell therapy has shown promise in the treatment of b-cell non-Hodgkin’s lymphoma and is currently being considered for patients with relapsed or refractory CD20-positive chronic lymphocytic leukemia.
Lym-1 targets conformational epitopes of human leukocyte antigen D-related antigens (HLA-DRs) on the surface of human B-cell lymphomas.
The binding affinity of Lym-1 to malignant B cells is higher than that of normal B cells, and Lym-1 CAR-T cells show a strong anti-tumor effect on B-cell lymphoma.
Some alternative targeting sites combined with CD19 to form dual-target CAR-T cells.
For example, combining CD37 and CD19 into one CAR generates bispecific CAR-T cells that can recognize both CD19 and CD37 individually or simultaneously.
CD79b is also a complementary targeting site for CD19.
CD19 and CD79 bispecific CAR-T cells prevent B-cell lymphoma escape from CD19 CAR-T cells.
Some alternative targeting sites have combined targeting functions, acting on tumor cells and the tumor microenvironment.
For example, CD123 is expressed in both Hodgkin’s lymphoma cells and tumor-associated macrophages, so CD123-resistant CAR-T cells can co-target both types of cells and kill them simultaneously.
CAR structures are constantly evolving to improve the efficacy of current CAR-T cell therapies.
Barriers to Current CAR-T Cell Therapy
From 2017 to 2021, the FDA has approved 5 CAR-T cell therapy products for marketing, as shown in the table below.
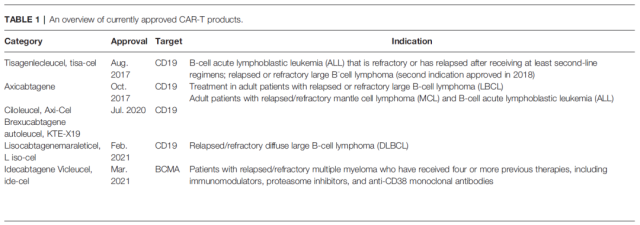 5 CAR-T cell therapy products approved by FDA
5 CAR-T cell therapy products approved by FDA
Kymriah™ (Tisagenlecleucel) is the first CAR-T cell therapy approved by the FDA for adult patients with certain types of B-cell lymphoma.
Three approved CAR-T cell products Yescarta™ (Axicabtagene ciloleucel) , TecartusE™ (Brexucabtagene autoleucel) and Breyanzi® (Lisocabtagene maraleucel) are also approved for the treatment of B-cell lymphoma.
The fifth CAR-T cell product, Abecma® (Idecabtagene vicleucel), is used for the treatment of multiple myeloma.
In addition to the 5 approved CAR-T cell products, a large number of CAR-T cells are being investigated in clinical trials, but current CAR-T therapy has some obstacles, such as associated toxicity, immunosuppressive tumor microenvironment and Complicated manufacturing processes, these obstacles hinder the wider application of CAR-T therapy.
Currently, the main toxicities associated with CAR-T therapy include cytokine release syndrome (CRS, also known as cytokine storm) , immune effector cell-associated neurotoxicity syndrome (ICANS) , and non-tumor-targeted toxicity.
CRS is caused by the production of a large number of inflammatory cytokines, such as IL-6, IL-10, IL-2, and TNFα, after CAR-T cell therapy. CRS often causes fever, hypotension, hypoxia, organ dysfunction, and even life-threatening adverse reactions.
The incidence of severe or life-threatening CRS can reach 25%. ICANS is another common toxicity associated with CAR-T cell therapy, characterized by neurological abnormalities and sequelae, and usually occurs within 1 week after CAR-T cell therapy.
Common adverse effects from ICANS include toxic encephalopathy with aphasia, confusion, and difficulty finding words.
Non-tumor on-target toxicity is due to the expression of the targeted protein on both normal and malignant cells, for example, when CD19 CAR-T cells are administered to a patient with malignant B cells, since CD19 CAR-T cells eradicate CD19+ B cell progenitors, non- Tumor targeting effects will lead to B cell aplasia and lead to hypogammaglobulinemia.
The immunosuppressive tumor microenvironment (MVT) inhibits the activation of CAR-T cells and accelerates the exhaustion of T cells.
The unfavorable factors of immunosuppressive MVT include hypoxia, various immunosuppressive cells, and persistent expression of co-inhibitory receptors.
Hypoxia was defined as hypoxia in tumor MVT. Immunosuppressive cells in tumor MVT include regulatory T cells (Tregs) , tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) .
The current manufacturing process of CAR-T cells is a very complicated work, including the collection of T cells, gene modification and expansion, and infusion back to patients.
These multi-step technologies and logistics are full of risks. Furthermore, the long and individualized manufacturing process poses great challenges for establishing standard operating procedures.
The current manufacturing process for CAR-T cells is expensive and technology-intensive, making them difficult to obtain for many cancer patients who need this new therapy.
Gene Editing Tools in CAR-T Cell Therapy
Gene editing tools commonly used in CAR-T cell therapy include ZFN , TALEN and CRISPR-Cas9 technologies. ZFN is the first generation of widely used gene editing tool, which can achieve effective and specific gene editing, but it takes a long time to optimize the target protein molecule.
As a second-generation gene editing tool, TALEN is more economical than ZFN, but it still takes a long time to optimize the system. CRISPR-Cas9 technology has become the most popular gene editing tool because of its simplicity, efficiency and specificity.
The development of gene editing technology represented by CRISPR-Cas9 has enabled the precise gene editing of CAR-T cells to generate anti-exhaustion T cells by removing PD-1 of T cells.
CRISPR-Cas9 has also been used to knock out endogenous antigens in normal cells, such as CD33 and CD7, to reduce non-tumor-targeted toxicity of redirected T cells.
At present, the CRISPR-Cas9 system has been used in CAR-T clinical trials of at least 21 target antigens, among which the two targets of CD19 and BCMA account for nearly half.
For broader use of the CRISPR-Cas9 system to edit CAR-T cells, efficient delivery methods must be developed.
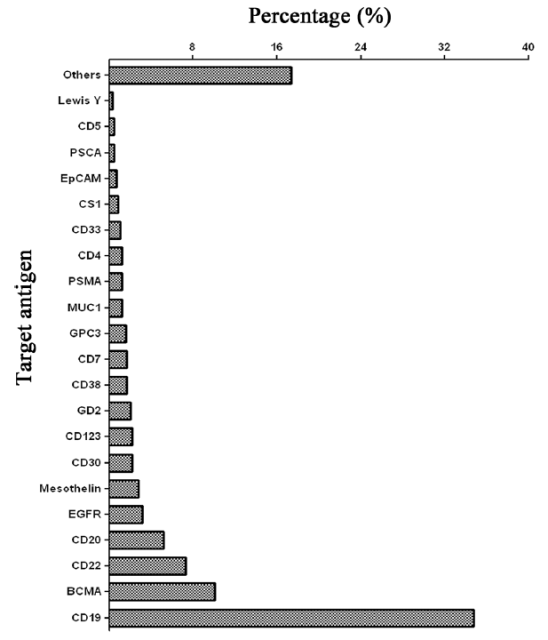
Targeted antigens for CAR-T cell therapy using CRISPR-Cas9 gene editing technology, data as of June 2021, only counting clinical trials registered in the United States
Gene delivery system
Several different delivery systems have been used to deliver gene therapy products, including the delivery of gene editing tools and CAR genes. Examples include various viral vectors and non-viral vectors.
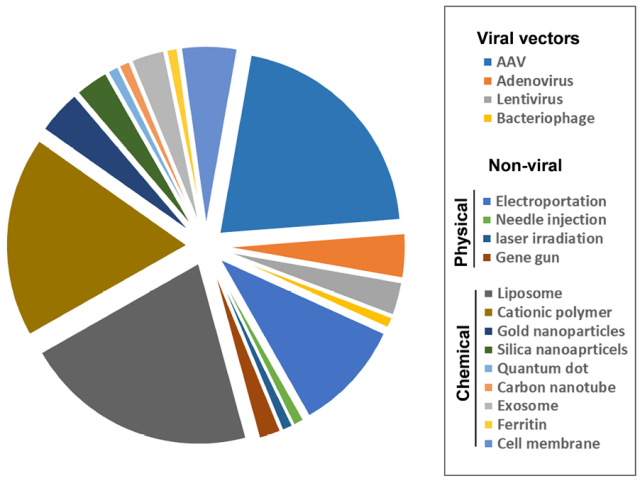 Classification and proportion of viral vectors and non-viral vectors used for gene delivery
Classification and proportion of viral vectors and non-viral vectors used for gene delivery
Viral vectors have the highest transfection efficiency and are widely used in gene delivery, but they have problems such as immunogenicity and cytotoxicity. Adeno-associated virus (AAV) has a lower risk of toxicity than other viral vectors (such as lentivirus and adenovirus ) , however, AAV vectors have a smaller load size than other viral vectors and can only deliver DNA fragments less than 5 kb.
In addition to viral vectors, there are also non-viral delivery systems, which can be divided into two categories : physical technology and chemical technology .
Physical techniques include electroporation, needle injection, laser irradiation, and gene guns.
Electroporation , one of the most widely used methods of gene delivery, uses electrical pulses to induce pore formation in cell membranes and transient permeability of genes.
Physical techniques are an attractive method of gene delivery due to their lower immunogenicity, but this method cannot target internal organs.
The chemical techniques used for gene delivery mainly include cationic lipid or polymer-based nanoparticles, gold nanoparticles, silica nanoparticles, carbon nanotubes, exosomes, ferritin, plasma membrane, etc.
Lipid-based nanoparticles are one of the most attractive nonviral gene delivery vehicles, and these vehicles have been approved for clinical use in several formulations.
In particular, lipid nanoparticles (LNPs) have been successfully used to deliver mRNA COVID-19 vaccines. LNP has also been used to deliver the CRISPR-Cas9 system, achieving potent gene editing and therapeutic effects in clinical trials.
Polymer-based nanoparticles are another suitable gene delivery system, and positively charged polymers can form stable multimers with genes that disrupt cell membranes and enable endosome escape.
A limitation of polymer-based nanoparticles is toxicity and immunogenicity resulting from the interaction of their positively charged surfaces with negatively charged cell membranes and proteins in the blood circulation.
Exosomes are naturally secreted nanometer-sized extracellular vesicles that are being extensively studied as gene delivery vehicles due to their natural biocompatibility and minimal immune clearance.
However, the use of exosomes as gene delivery vectors requires overcoming difficulties in production, isolation, and purification, and there are still many challenges.
Plasma membranes from platelets and red blood cells are biomimetic carriers for gene delivery, which have natural biocompatibility and targeting, but their transfection efficiency needs to be improved.
Other chemical nanocarriers all have their own properties that determine their impact on gene delivery.
Some have shown potential efficacy in the treatment of various diseases, but the optimal drug delivery system has not yet achieved clinical application.
In vivo CAR-T cell induction
The current manufacturing process of CAR-T cells requires specialized equipment and a large amount of technology accumulation, and is labor-intensive and time-consuming, which limits the widespread application of this technology globally and pushes up CAR-T cell therapy. The price is unaffordable for many patients.
In order to simplify the production process, universal CAR-T cells derived from allogeneic healthy people have been tested in clinical trials, however, due to safety concerns about allogeneic CAR-T cells, the FDA recently suspended universal CAR-T cells derived from allogeneic genes – Clinical trials of T cells.
Therefore, there is an urgent need to develop a safe and simple CAR-T cell production process.
In vivo programming of CAR-T cells via nanoparticles is an elegant and novel approach to simplify and standardize the complex manufacturing process of CAR-T cells in vitro.
In addition, in situ induction of CAR-T cells can effectively reduce systemic toxicity such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) .
As early as 2017 and 2020, Professor Matthias Stephan of the Fred Hutchinson Cancer Research Center in the United States published papers in the journals of Nature Nanotechnology and Nature Communications [2, 3] .
CAR-T cells were induced in vivo by delivering CAR-DNA or CAR-mRNA via nanocarriers.
In both works, the core of the nanodelivery system was constructed from the cationic polymer poly(β-aminoester) and assembled with a second-generation CAR construct targeting CD19.
The exterior of the nano-delivery system is made of polyglutamic acid (PGA) conjugated with anti-CD3 antibody.
The polymer nanoparticles carrying the CD19-specific CAR gene rapidly and specifically edited T cells in vivo, and brought anti-tumor effects comparable to those of conventional laboratory-made CAR-T cells without causing systemic toxicity.
Christian Buchholz et al. reported for the first time that a second-generation anti-CD19-CAR gene-wrapped lentivirus induced CAR-T cells in situ in immunodeficient NOD-scid-IL2Rc null (NSG) mice and showed antitumor activity.
In their study, CAR-positive NK and NKT cells were detected unexpectedly, which may be due to the non-specificity of the lentiviral vector.
In order to overcome the non-specificity of viral vectors, Samuel K Lai et al. developed a bispecific binding agent to redirect lentiviral vectors to T cells for in vivo specific engineering of CAR-T cells.
They observed that in vivo CAR-T cells engineered by lentivirus had anti-tumor activity, but the number of T cells expressing CAR was relatively small.
They believe this demonstrates a valuable, unproven theory that in vivo CAR-T cells have better performance and self-renewal capabilities than ex vivo CAR-T cells.
Among viral vectors, adeno-associated virus (AAV) has a lower risk of toxicity.
In March 2021, Wu Xilin and others from Nanjing University School of Medicine published a paper in Blood Cancer Journal [4] , reporting that the third-generation CAR gene encoded by AAV can Sufficient reprogramming of immune effector cells to generate CAR-T cells in vivo.
In this work, they demonstrated the concept of AAV inducing CAR-T cells in vivo, but they also expressed concerns about the non-specificity of AAV carrying the CAR gene.
In addition to the non-specificity of viral vectors, another general safety concern of viral vectors is that they may lead to random insertion of genes in chromosomes.
Precise and rapid gene editing tools such as CRISPR have been widely used to generate CAR-T cells in vitro.
There are many studies on in vivo gene editing using CRISPR, but there is no report on the application of CRISPR to generate CAR-T cells in vivo.
Future applications combining gene editing tools and CAR genes will accelerate the clinical application of CAR-T cells in vivo.
Nanocarrier delivery designed CAR-structure and gene editing tools can induce the formation of CAR-T cells with multiple functions in vivo to overcome the obstacles of current CAR-T cells, such as related toxic effects, immunosuppressive microenvironment and complex manufacturing process.
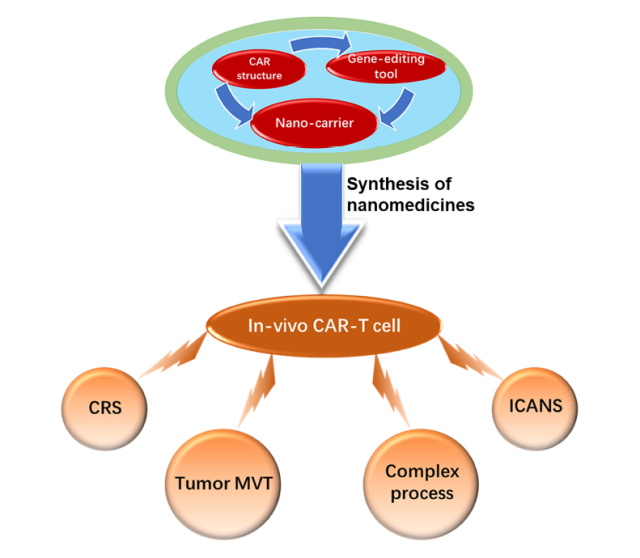
Systemic toxic effects can be reduced by in situ editing of tumors and expansion of T cells.
Adding special cytokine genes to the CAR structure enables CAR-T cells to secrete cytokines and improve the immunosuppressive microenvironment, making it suitable for the survival and proliferation of T cells.
Loading the gene-editing tool of the CAR construct into the nanoparticles can knock out immune checkpoint genes, thereby reversing T cell exhaustion.
More importantly, this method solves the problem of standardization and scale-up of the in vitro CAR-T cell manufacturing process.
Ultimately, CAR-T cell therapy products can be conveniently produced, stored, and used as conventional drugs through nano-delivery vectors.
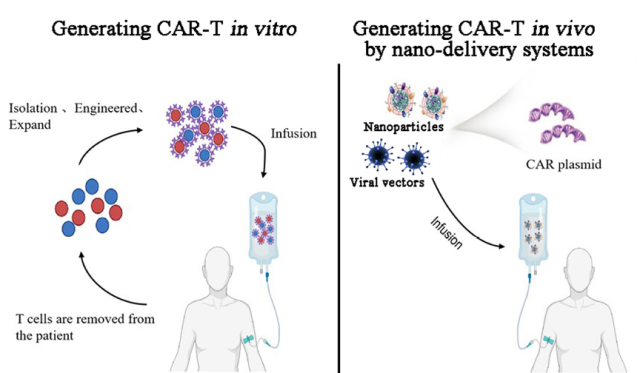 Comparison of in vitro generation of CAR-T and in vivo generation of CAR-T based on nano-delivery system
Comparison of in vitro generation of CAR-T and in vivo generation of CAR-T based on nano-delivery system
The above studies are just the beginning of the in situ induction of CAR-T cells in vivo. To achieve clinical application, more efforts are needed to monitor the editing and expansion status of T cells in vivo.
Conclusion and Outlook: Inducing CAR-T cells in vivo expected to break through the barriers of current cell therapy
In the past decade, CAR-T cell therapy has made great achievements, and five CAR-T cell products have been approved by the FDA.
However, current CAR-T cell therapy also has some obstacles to overcome, such as the toxicity of CRS and ICANS and expensive and complicated manufacturing procedures.
Induction of CAR-T cells in vivo by CAR gene-loaded nanoparticles and gene editing tools has shown a potential breakthrough in overcoming current barriers to CAR-T cell therapy.
Although few studies have reported nanoparticle-induced CAR-T cells in vivo, reliable preclinical data predict the future prospects of cell therapy via nanodelivery methods.
The field of in vivo induction of CAR-T cell therapy is still in its infancy, and translating this approach into clinical practice also faces many challenges. Summary of the System Nano-delivery systems for inducing CAR-T cells in vivo can guide the design of nanoparticles and their payloads to optimize their efficacy.
In summary, in vivo induction of CAR-T cells is expected to replace the current CAR-T cell therapy and become the standard immune cell therapy for cancer treatment.
Paper link :
1. https://www.frontiersin.org/articles/10.3389/fonc.2022.809754/full
2. https://www.nature.com/articles/nnano.2017.57
3. https://www.nature.com/articles/s41467-020-19486-2
4. https://www.nature.com/articles/s41408-021-00508-1
Inducing CAR-T cells in vivo expected to break through the barriers of current cell therapy
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



