Does COVID-19 Monoclonal Antibody Therapy Really Work?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Does COVID-19 Monoclonal Antibody Therapy Really Work?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Does COVID-19 Monoclonal Antibody Therapy Really Work?
A study by UPMC and the University of Pittsburgh School of Medicine found that monoclonal antibody therapy significantly reduced the risk of hospitalization or death from COVID-19.
The research highlights the importance of investing in infrastructure and healthcare expertise to deliver these treatments effectively, especially if more deadly virus variants emerge in the future.
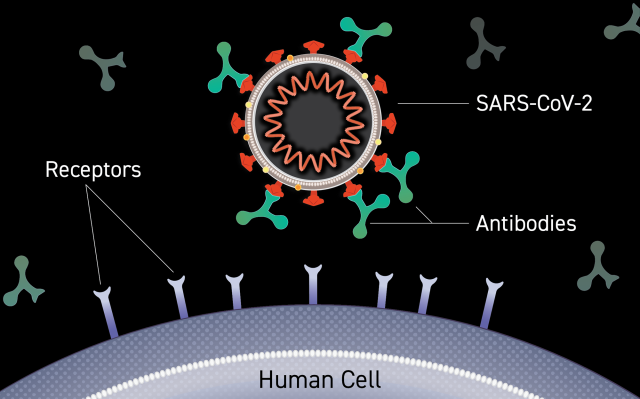
Image source: www.cancer.gov
The COVID-19 pandemic has created a true experimental environment, requiring healthcare providers across the country to rapidly establish clinics to administer the evolving monoclonal antibody therapy, which, while initially shown to be safe and effective in clinical trials, and in It is approved under a federal emergency use authorization, but it has never been tested on such a large scale.
The task is enormous, and the real-world benefit is uncertain, especially for monoclonal drugs approved in the late stages of the pandemic based solely on laboratory data. It’s worth it, according to an analysis published today (April 3, 2023) in the Annals of Internal Medicine by clinicians and scientists at UPMC and the University of Pittsburgh School of Medicine.
“The virus is a moving target, and over a two-year period, monoclonal antibody therapies were approved by regulators, withdrawn, sometimes reauthorized, and sometimes scarce,” said lead author Dr. Kevin Kip, vice president of clinical analysis at UPMC . “Using UPMC’s monoclonal antibody-treated patient database — one of the largest in the United States — we were finally able to conclude that driven by all of these challenges, lives were definitively saved while preventing inpatient departments from being overwhelmed.”
Monoclonal antibodies are human-made antibodies specifically designed to protect against pathogens — in this case, the virus that causes COVID-19 — to enter human cells, replicate and cause severe disease.
The U.S. Food and Drug Administration granted emergency use authorizations to five different COVID-19 monoclonal antibody treatments at various times between 2020 and 2022.
All treatments are restricted to those 12 years and older with risk factors that make them more susceptible to adverse outcomes from COVID-19.
These treatments must be given intravenously or given by a healthcare provider. As the virus evolves, new mAbs are introduced and old mAbs that are no longer effective are removed.
Since its first authorization in late 2020, UPMC has opened dozens of clinics, built emergency department infrastructure, and scheduled home visits to maximize delivery of mAbs to patients in Pennsylvania, New York, and Maryland Ability. After the emergency use authorization of the last monoclonal antibody was revoked on November 30, 2022, there will be no new monoclonal antibodies.
The data of 5135 COVID-19 patients who were eligible for monoclonal antibody treatment but did not receive treatment were compared.
On average, people who received monoclonal antibody treatment within two days of testing positive for COVID-19 had a 39 percent lower risk of hospitalization or death compared with their peers who did not receive treatment. Immunocompromised patients, regardless of age, had an even greater reduction in risk.
Patients who were treated when alpha and delta variants of the virus were circulating experienced a greater benefit than their untreated peers, likely because earlier variants were more lethal and people had previously been infected or vaccinated Immunity is lower after the vaccine.
Co-author Erin McCreary, MD, director of infectious disease improvement and clinical research innovation at UPMC, explained that by the time omicrons circulated, the risk of death and hospitalization had generally declined, so the overall benefit of monoclonal antibody therapy was lower but still clinically meaningful, especially for vulnerable patients.
“Right now, the risk of death from COVID-19 to the general population is relatively low, but we’ve seen how quickly the virus can mutate and spread. No one can say for sure that future variants won’t be more deadly,” McCreary said. “If that happens, our real-world data reassures that investing in the infrastructure and knowledge of healthcare workers to deliver antibody treatments quickly will keep people alive and out of hospital in the communities we serve.
Reference:
https://www.cancer.gov/news-events/cancer-currents-blog/2021/covid-19-antibodies-nci-seronet
Does COVID-19 Monoclonal Antibody Therapy Really Work?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



