Cytokine release syndrome and related neurotoxicity in CAR-T therapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Cytokine release syndrome and related neurotoxicity in CAR-T therapy.
Adoptive immunotherapy is a revolutionary treatment method in hematology. It expresses synthetic chimeric antigen receptor (CAR) in T cells through genetic engineering.
CAR-T cells targeting the CD19 antigen in B-cell leukemia and lymphoma have been clinically recognized, and are now a “routine” treatment implemented by cancer centers worldwide.
However, the clinical benefits of CAR-T observed in many patients may come at a price. In up to one-third of patients, significant toxicity is directly related to the induction of a strong immune response. The most common immune-mediated toxicity is cytokine release syndrome (CRS) and immune effector cell-related neurotoxicity syndrome (ICANS).
CRS usually starts with fever and physical symptoms, such as stiffness, malaise, and anorexia. In severe cases, CRS manifests as other features of the systemic inflammatory response, including hypotension, hypoxia, and/or organ dysfunction. However, if the symptoms and signs of CRS are recognized and treated in time, the organ dysfunction in most patients can be prevented or reversible.
ICAN usually manifests as toxic encephalopathy. The first is to find difficulty in vocabulary, confusion, dysplasia, aphasia, impaired fine motor skills, and lethargy. In more severe cases, seizures, motor weakness, cerebral edema, and coma have been detected.
Most patients with clinical features of ICAN will have symptoms before CRS. Therefore, CRS can be regarded as the “initiating event” or cofactor of ICAN. ICAN usually occurs after CRS symptoms subsided. Similar to CRS, ICAN is reversible in most patients without permanent neurological dysfunction.
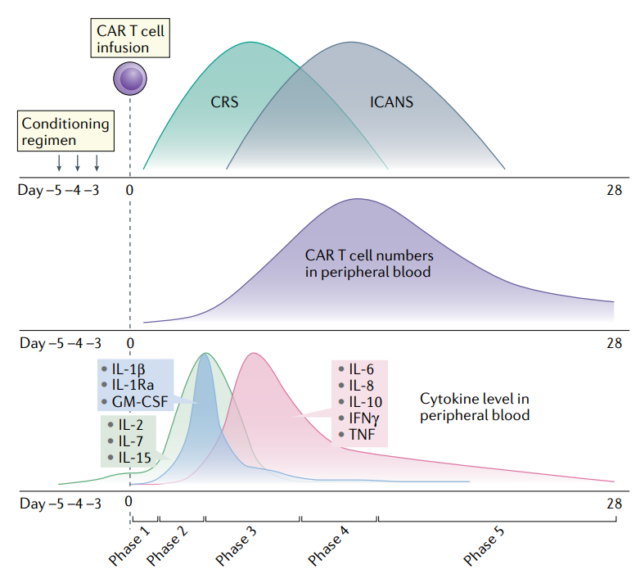
Pathophysiology of CRS
The pathophysiology of CRS can be divided into five main stages:
- First stage, CAR-T cells are transported to the tumor site after infusion into the patient’s body, and CAR-mediated antigen expression target cell recognition;
- Second stage, CAR-T cells at the tumor site proliferate, activated CAR-T cells and cellular components of the tumor microenvironment produce cytokines in situ, activate the “bystander” endogenous immune cells, and kill the tumor directly and indirectly Cells, and CRS occurs;
- Third stage, the level of cytokines increases, and the CAR-T cell population in the peripheral blood increases, which is related to the systemic inflammatory response. This leads to endothelial damage and vascular leakage in multiple tissues and organs, and their related effects, including hypoxia, hypotension, and/or organ damage.
- Fourth stage, cytokines spread, CAR-T cells, endogenous T cells and peripherally activated monocytes enter the cerebrospinal fluid (CSF) and central nervous system (CNS), including the destruction of the blood-brain barrier (BBB), and The onset of ICANS is consistent.
- Fifth stage, activation-induced T cell death after tumor clearance leads to a decrease in serum cytokine levels and a reduction in systemic inflammatory response, the end of CRS and/or ICANS symptoms, and the persistence of long-term memory T cells.
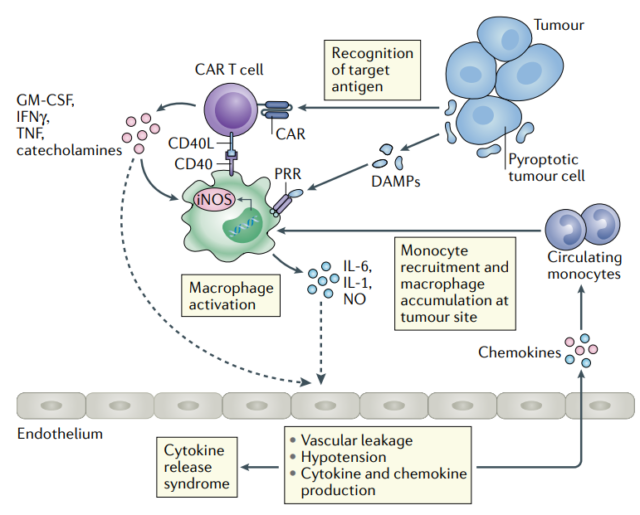
Cell interaction
Animal models show that CRS is caused by a multicellular network, which includes CAR-T cells and host cells, with macrophages and monocytes. It is currently uncertain whether a contact-dependent interaction is required between CAR-T cells and host myeloid cells.
Although the CD40-CD40L interaction is not necessary to induce CRS, it can aggravate the activation of macrophages, thereby increasing the production of IL-6 and exacerbating CRS.
In addition, given the bidirectional signal and potential role of the CD28–CD80/CD86 axis in inducing IL-6 production by myeloid cells, the CD28–CD80/CD86 axis may be particularly important in CRS.
Blocking these interactions may reduce the severity of CRS.
IL-6 and IL-1
The cytokines IL-6 and IL-1 play an important role in the pathophysiology of CRS. IL-6 is a pleiotropic cytokine with pro-inflammatory and anti-inflammatory effects.
IL-6 is mainly produced by macrophages and other myeloid cells, and can act in an autocrine manner, and together with other inflammatory signals, it promotes the maturation and activation of macrophages.
In many patients, IL-6 blockade can lead to the reversal of most symptoms and the overall down-regulation of cytokines.
IL-1 is also a pleiotropic cytokine with multiple functions. Mainly produced by monocytes and macrophages. IL-1 can induce tissues to produce downstream pro-inflammatory cytokines, such as IL-6, and a series of chemokines for maturation and recruitment of tissue immune cells.
In addition, IL-1 can activate the production of pro-inflammatory lipid mediators (such as prostaglandin E2, which can promote edema), induce acute-phase proteins and send signals to the hypothalamus, pituitary and adrenal glands, and have direct and indirect effects on the circulatory system.
Damage-related molecules and other soluble media
The release of tumor cell damage-related molecules can cause macrophages to activate in vitro to produce IL-6 and IL-1.
Granulocyte-macrophage colony stimulating factor (GM-CSF) is produced by a variety of cell types, including activated CAR-T cells. Blocking it can eliminate the production of IL-6 and other cytokines by monocytes in vitro. However, GM-CSF may still be a precipitating factor of CRS, rather than a necessary condition for CRS.
Tumor Necrosis Factor (TNF) is another pleiotropic cytokine that mediates inflammation by activating T cells, macrophages, monocytes, and other cells of the immune system. Compared with IL-1 and IL-6, TNF also has direct anti-tumor activity.
In recent years, TNF has been considered as a potential factor for the induction of CRS by CD3 and HER2 bispecific antibodies in the HER2 humanized mouse breast cancer virus (MMTV) model.
Interferon-γ (IFN-γ) can be produced in large quantities by activated T cells. It is induced by iNOS to promote the maturation of macrophages and promote the production of NO. In addition, IFNγ can promote the permeability of other tissues (including BBB) by loosening tight junctions.
The adrenal system is also involved in the development and maintenance of CRS, because catecholamine epinephrine and norepinephrine directly affect the activation of CAR-T cells and subsequent cytokine release.
Pathophysiology of ICANS
Although the clinical features of ICANS are easy to identify, their pathophysiology is still poorly understood.
Recent animal models indicate that the activation of endothelial cells and the destruction of the blood-brain barrier lead to direct nerve cell damage, as well as the effects of various pro-inflammatory cytokines.
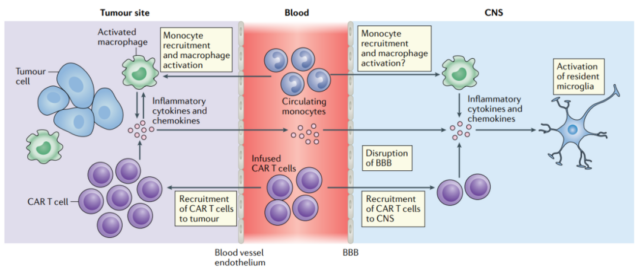
Vascular permeability, endothelial cell destruction and glial cell damage
The levels of protein, CD4+T cells, CD8+T cells, and CAR-T cells in the cerebrospinal fluid of ICANS patients increased, indicating that the integrity of the BBB was lost.
Clinical studies have shown that there is a correlation between the number of CAR-T cells in the cerebrospinal fluid and the level of cytokines and the severity of ICANS.
In addition to the destruction of BBB and the increase in vascular permeability, children and young adult patients also reported glial cell damage in patients who developed ICANS after CD19 CAR-T cell therapy.
The levels of GFAP and S100b in the cerebrospinal fluid of patients with acute neurotoxicity are significantly increased. GFAP is a good marker of astrocyte damage, and S100b in CSF is a marker of astrocyte activation.
Cytokines
Many clinical trials have shown that there is an association between the increase in serum levels of various cytokines and the risk of ICANS. Cytokines that continue to increase in multiple studies (using various CAR structures and different targeted malignancies) include IL-2, IL-6, IL-10, and IL-15.
However, although the mouse model suggests that monocyte-derived immune cells play a significant role in cytokine secretion and the pathogenesis of CRS and ICANS, it is still impossible to determine the specific source of cytokines in ICAN patients in clinical trials.
In addition, the number of myeloid cells is significantly increased in the cerebrospinal fluid of patients with severe ICANS.
The expression of target antigen in the central nervous system
Data from clinical trials indicate that the presence of antigen-positive tumor cells in the central nervous system is not necessary for the occurrence of ICANS.
However, in a recent study, single-cell RNA sequencing analysis showed that CD19 is expressed in human brain wall cells, including pericytes and vascular smooth muscle cells, which increases the targeted non-tumor effect and may be associated with CD19 CAR-T cells The possibility of neurotoxicity.
This observation may explain the higher incidence of ICANS observed in CD19 targeted therapy compared with targeted CD20, CD22 and BCMA therapy.
Brain edema
Cerebral edema is a rare and potentially fatal neurological complication that has been observed after CAR-T cell therapy.
Existing evidence suggests that the pathophysiology of cerebral edema may be different from the common encephalopathy manifestations of ICANS.
Autopsy of patients with fatal brain edema after CAR-T treatment showed that the blood-brain barrier was completely destroyed, but the central nervous system lacked activated T cells.
This suggests that the destruction of the blood-brain barrier and subsequent brain edema may be due to the surge of inflammatory cytokines, rather than the infiltration of CAR-T cells into the central nervous system.
Clinical management of CRS and ICANS
Low-grade CRS is managed with supportive treatment with antipyretics, while ensuring that there are no concurrent causes of fever, such as infection.
Moderate to severe CRS is treated with IL-6R blocking antibody tocilizumab, with or without glucocorticoids for immunosuppression, and intensive supportive treatment.
Although tocilizumab is very effective in the treatment of CRS, it has no effect in most ICANS cases. This may be due to the pathophysiological difference between CRS and ICANS and/or the poor permeability of tocilizumab to BBB.
In fact, prophylactic use of tocilizumab reduces the incidence of severe CRS, but increases the incidence of severe ICANS, which may be due to the increased serum IL-6 levels observed after tocilizumab administration.
In addition, most studies have shown that the use of tocilizumab does not seem to affect the efficacy of CAR-T cells.
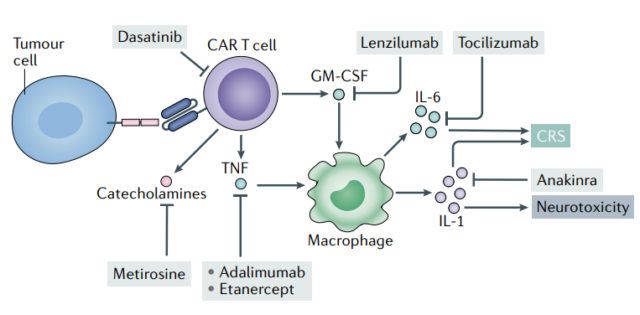
Non-specific immunosuppressants, such as corticosteroids, can relieve symptoms in many patients.
In addition, other broad-spectrum cytokine inhibitors, such as Ruxotitinib, block JAK1 and JAK2 or Itatinib, and blocking JAK1 may attenuate the effects of pro-inflammatory cytokines such as IFN-γ and IL-6.
Similarly, ibrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor, also inhibits IL-2 tyrosine kinase (ITK), resulting in decreased cytokine secretion in the mouse model, including IL-6.
However, non-specific immunosuppressive agents may also directly affect the activation level of CAR-T cells, thereby affecting clinical efficacy.
Dasatinib is a BCR ABL targeted kinase inhibitor, approved for the treatment of various hematological malignancies. It effectively inhibits CAR-T cell-mediated cytotoxicity and cytokine production in a rapid and reversible manner.
In addition, short-term administration of Dasatinib can reduce CRS-related mortality, but does not affect the anti-tumor effect in the body, and can restore CAR T cell activity after stopping the drug.
In addition, the IL-1R inhibitor anakinra is beneficial to nearly half of patients with severe ICANS after CAR-T treatment.
Influence of other clinical factors
The occurrence, severity and duration of CRS and ICANS after CAR-T cell therapy may be affected by the host, tumor and/or treatment-related factors.
Patients with high baseline inflammatory status (such as C-reactive protein, ferritin, D-dimer, and pro-inflammatory cytokine levels) are at increased risk of CRS and ICANS.
More severe cases of CRS and/or ICANS are also related to a higher tumor burden in multiple malignancies, which may be related to the expansion and simultaneous activation of CAR-T cell populations.
In addition, the older the age, the higher the risk of severe CRS or ICANS.
The impact of CAR design
Recent studies have shown that the design of the CAR molecule itself can significantly affect the proliferation of CAR-T cells and the distribution of cytokines, thereby affecting the incidence and severity of CRS and/or ICANS.
The CAR-T cell product with the CD28 costimulatory signal domain appears to proliferate faster after infusion, and its number seems to peak earlier than the product with the 4-1BB costimulatory signal domain.
In clinical studies, this is related to the earlier and higher incidence of more severe CRS and ICANS. However, so far, there has not been a direct comparison between CAR-T cell products with CD28 and 4-1BB costimulatory domains.
In addition, evidence in the literature also shows that changing the non-signaling domains of CAR molecules, including the hinge and transmembrane region or antigen binding domain (scFv), may also affect the toxicity associated with CAR-T cell therapy.
Outlook
At present, the wide application of CAR-T cell therapy is limited to a certain extent. This is due to the occurrence of CRS and ICANS in the treatment and the resulting burden.
An in-depth understanding of the molecular and cellular pathophysiology of CRS and ICANS will help develop effective targeted therapies that reduce toxicity without affecting anti-tumor activity.
At present, the new CAR structure has been designed to minimize the risk of inducing CRS and ICANS, while optimizing tumor antigen recognition and effective T cell signaling.
How to effectively and reliably prevent these complications in the future will be one of the key factors for the success of CAR-T cell therapy.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



