A cytokine storm appeared after a cancer patient received mRNA vaccine
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
First discovery: A cytokine storm appeared after a cancer patient received COVID-19 mRNA vaccine
A cytokine storm appeared after a cancer patient received mRNA vaccine. Cytokine Release Syndrome (Cytokine Release Syndrome, CRS), also known vividly as Cytokine Storm (Cytokine Storm), is usually a systemic inflammatory response that causes the body to release a large amount of cytokines due to microbial infection, which causes acute respiratory distress Syndrome and important causes of multiple organ failure.
In addition to microbial infections such as bacteria and viruses, CAR-T cell therapy is also prone to cytokine storms. In addition, cytokine storms may also occur during immune checkpoint inhibitor therapy, but the probability is extremely low.
In the COVID-19 pneumonia vaccination plan, cancer patients are the priority targets for vaccination, and there is no cytokine storm caused by vaccination.
On May 26, 2021, researchers from the Francis Crick Institute in the United Kingdom published a research paper titled: Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2 in the top international medical journal Nature Medicine.
The study found that a patient with colorectal cancer who received PD-1 therapy for a long time experienced a cytokine storm after being vaccinated with the mRNA COVID-19 vaccine developed by Pfizer/BioNTech.

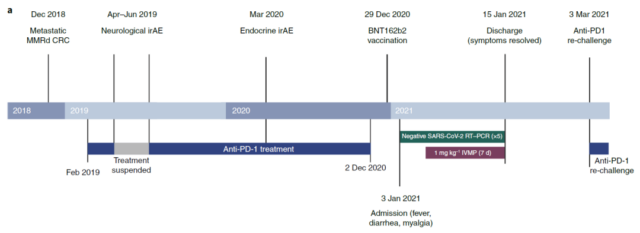
This 58-year-old male patient started monotherapy with anti-PD-1 monoclonal antibody in February 2019 to treat his metastatic mismatch repair defective colorectal cancer.
Two months after the start of treatment, he experienced neuroimmune-related adverse reactions (irAE) against the background of preexisting spinocerebellar ataxia. The ataxia worsened, but the cause is unknown, so the anti-PD-1 monoclonal antibody treatment was suspended. Then I started taking prednisolone and my ataxia recovered. In June 2019, the patient restarted anti-PD-1 monoclonal antibody treatment and his condition was stable. In March 2020, the patient developed an immune-related adverse reaction (irAE) and took prednisolone again, and disease control was maintained.
On December 2, 2020, the patient received anti-PD-1 monoclonal antibody treatment for the last time. On December 29, 27 days later, the patient was vaccinated with the mRNA COVID-19 vaccine BNT162b2 developed by Pfizer/BioNTech. The patient had not been infected with the new coronavirus before, and there was no immediate adverse reaction after vaccination.
Five days after vaccination, on January 3, 2021 (32 days after the last anti-PD-1 monoclonal antibody treatment), despite taking ibuprofen, the analgesic drug, the patient still developed muscle pain, diarrhea and Fever symptoms.
Broad-spectrum antibiotic treatment and related tests, and SARS-CoV-2 RT-PCR test showed that the patient was not infected with the virus, nor was he infected with the new coronavirus.
For the next 5 days, the patient continued to have a fever of up to 39.8 °C, thrombocytopenia, increased inflammation markers, and a significant increase in ferritin.
After admission, the patient was suspected to have a grade 3 cytokine storm and received intravenous methylprednisolone (IVMP) hormone therapy. All indicators began to return to normal after 7 days. After being discharged from the hospital and returning home, the patient did not have fever and other symptoms and remained in good condition.
On February 8, 2021 (36 days after the initial visit), the patient received anti-PD-1 monoclonal antibody treatment again without any adverse events. He did not receive a second dose of mRNA vaccine.
In the context of anti-PD-1 monotherapy, less than 0.01% of immune-related adverse reactions (irAE) involve cytokine storm, and so far, two large-scale vaccination mRNA vaccines (BNT162b2, mRNA-1273) have not The report causes a cytokine factor storm.
The cytokine storm associated with immune checkpoint blocking therapy usually occurs in the 4th week after treatment, and the patient has started anti-PD-1 monoclonal antibody immune checkpoint blocking therapy 22 months ago, which makes the treatment not It may be the only reason for its cytokine storm.
In this case, the close temporal correlation between mRNA vaccination and its clinical manifestations of cytokine storm suggests that mRNA vaccine may be a potential trigger of cytokine storm.
The author of the paper stated that cancer patients were excluded from the research during the development of the COVID-19 vaccine, but cancer patients were included in the vaccination. Therefore, the safety of the COVID-19 vaccine for cancer patients has not actually been fully studied. The case also reminded the prospective pharmacovigilance on the safety of the COVID-19 vaccine in cancer patients.
Finally, the author of the paper stated that the conditions in the paper are just one case, not universal, and cancer patients are usually more likely to be infected with the COVID-19 virus, and the benefits of cancer patients vaccinated against the COVID-19 far exceed the risks. Therefore, it is still recommended that cancer patients receive the COVID-19 vaccine in time
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



