Innovative treatment for childhood brain tumors: Phase II trials of Tovorafenib is successful
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Innovative treatment for childhood brain tumors: Phase II trials of Tovorafenib is successful
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Innovative treatment for childhood brain tumors: Phase II trials of Tovorafenib is successful, The overall response rate was 64%.
On June 13, Day One Biopharmaceuticals announced positive results from a pivotal Phase 2 clinical trial of its brain-penetrating, oral specific pan-RAF kinase inhibitor tovorafenib in patients with pediatric low-grade glioma (pLGG).
Among the first 22 evaluable patients, tovorafenib achieved an overall response rate (ORR) of 64%.
Top-line results from this trial are expected in the first quarter of next year, and if the results are positive, the company plans to submit a regulatory filing in the first half of next year.
Pediatric low-grade gliomas are the most common brain tumors in children, accounting for approximately 30–50% of all CNS tumors.
In pLGG patients, BRAF gene fusions are the most common cancer driver.
Currently approved BRAF inhibitors are only active against tumors with BRAF V600 mutations, have limited activity in brain tumors, and cannot be used in patients with BRAF fusions.
Tovorafenib is a highly specific pan-RAF kinase inhibitor that inhibits the growth of tumors harboring BRAF fusions or BRAF V600 mutations and is brain-penetrant.
It has received Breakthrough Therapy Designation and Rare Pediatric Disease Designation from the US FDA for the treatment of pLGG with activating RAF variants.
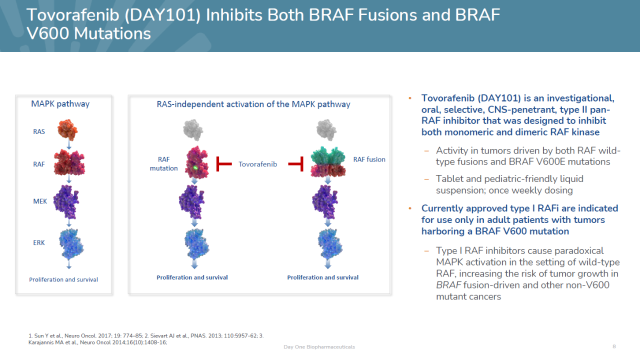
▲Introduction to Tovorafenib (Image source: Day One Biopharmaceuticals’ official website)
Trial data showed that in the first 22 patients evaluable for efficacy, tovorafenib achieved an ORR of 64%, including 14 patients with partial responses.
At the same time, 6 patients had stable disease, and the disease control rate was 91%.
All patients with stable disease also experienced tumor shrinkage, ranging from 19% to 34%. Responses were observed in both treatment-experienced patients with BRAF fusions or BRAF V600E mutations.
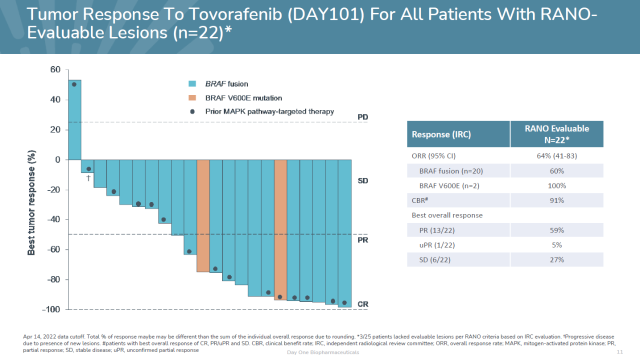
▲The efficacy data of Tovorafenib (Image source: Day One Biopharmaceuticals official website)
In terms of safety, tovorafenib was well tolerated based on data from 25 patients. Major adverse events were grade 1 or 2. Grade 3 or higher treatment-related adverse events occurred in 36% of patients.
“These preliminary studies show the potential of tovorafenib monotherapy for the treatment of relapsed/progressed pLGG,” said Samuel Blackman, MD, Co-Founder and Chief Medical Officer of Day One. “Our registrational study has completed enrollment of all patients and follow-up is ongoing. We expect to have top-line results from all participants in the first quarter of 2023. Based on these positive preliminary data, we plan to initiate a pivotal Phase 3 clinical trial evaluating tovorafenib as a first-line treatment for pLGG.”
References:
[1] Day One Announces Positive Initial Data from Pivotal FIREFLY-1 Trial of Tovorafenib (DAY101) in Relapsed Pediatric Low-Grade Glioma. Retrieved June 13, 2022, from https://ir.dayonebio.com/news-releases/news -release-details/day-one-announces-positive-initial-data-pivotal-firefly-1-trial
Innovative treatment for childhood brain tumors: Phase II trials of Tovorafenib is successful, The overall response rate was 64%.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



