New drug for ulcerative colitis: AbbVie Rinvoq Phase 3 Trials Successful
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New drug for ulcerative colitis: AbbVie Rinvoq Phase 3 Trials Successful
New drug for ulcerative colitis: AbbVie Rinvoq Phase 3 Trials Successful. New drug for ulcerative colitis (UC)! AbbVie’s oral JAK1 inhibitor Rinvoq Phase 3 maintenance study was successful: the primary endpoint and all secondary endpoints were reached!
AbbVie recently announced the positive results of a phase 3 maintenance study (NCT02819635) evaluating a new anti-inflammatory drug oral JAK1 inhibitor Rinvoq (upadacitinib) in the treatment of moderate to severe active ulcerative colitis (UC). The data showed that two doses of Rinvoq (15mg and 30mg, once a day) reached the primary endpoint (clinical remission) and all secondary endpoints after one year of treatment (52 weeks). At week 52, compared with the placebo group, a significantly higher proportion of patients in the Rinvoq treatment group achieved clinical remission (42% in the 15 mg group, 52% in the 30 mg group, and 12% in the placebo group; p <0.001).
Ulcerative colitis (UC, image source: healthjade.com)
In the study, after an 8-week study period of upadacitinib (45mg) once-a-day induction therapy, patients with moderate to severe UC who obtained a clinical response were randomly assigned to receive upadacitinib 15mg, 30mg, and placebo for 52 weeks .
The data showed that at week 52, the study reached all secondary endpoints, including endoscopic improvement, histological endoscopic mucosal improvement (HEMI), and clinical remission without corticosteroids. The specific data are: (1) In the 52nd week, 49%, 62%, and 14% of the patients in the upadacitinib 15mg group, 30mg group, and placebo group achieved endoscopic improvement (p<0.001); (2) In the 52nd week Weeks, 35%, 49%, and 12% of the patients in the upadacitinib 15mg group, 30mg group, and placebo group achieved HEMI (p<0.001); (3) Among the patients who were in remission at the completion of the 8-week induction study, in the first At 52 weeks, 57%, 68%, and 22% of patients in the upadacitinib 15mg group, 30mg group, and placebo group achieved corticosteroid-free remission (p<0.001).
In this study, the safety results of upadacitinib (15mg, 30mg) are consistent with the safety characteristics observed in the phase 3 induction study of ulcerative colitis and previous clinical studies for other indications. No new security risks have been discovered. During the 52-week study period, the most common adverse events observed in the upadacitinib group were nasopharyngitis, exacerbation of ulcerative colitis, and elevated blood creatine phosphokinase levels.
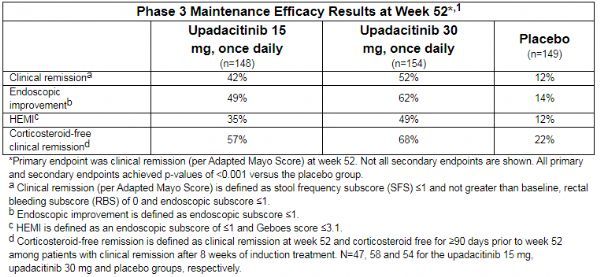
The 52-week efficacy results of the Phase 3 maintenance study
All results of the Phase 3 maintenance study will be announced at future medical conferences and submitted to peer-reviewed journals for publication. The main results of phase 3 induction studies (U-ACHIEVE and U-ACHIEVE) were announced in December 2020 and February 2021, respectively. The use of upadacitinib in ulcerative colitis (UC) has not been approved, and its safety and effectiveness have not been evaluated by regulatory agencies.
Professor Remo Panaccione, Director of the Inflammatory Bowel Disease (IBD) Unit at the University of Calgary, said: “Ulcerative colitis is a challenging disease. Many patients still do not get relief from the most onerous symptoms. These positive results indicate that in At 52 weeks, upadacitinib improved clinical, endoscopic and histological results. This is good news for the IBD community.”
Dr. Michael Severino, Vice Chairman and President of AbbVie, said: “Ulcerative colitis is a disease with unpredictable symptoms and frequent episodes, which poses challenges to daily life. These results show that upadacitinib is a moderately severe ulcerative We are encouraged by the potential of patient treatment options.”
The active pharmaceutical ingredient of Rinvoq is upadacitinib, which is an oral selective and reversible JAK1 inhibitor discovered and developed by AbbVie. It is being developed to treat several immune-mediated inflammatory diseases. JAK1 is a kinase that plays a key role in the pathophysiology of many inflammatory diseases.
Rinvoq is an oral, once-daily, selective and reversible JAK inhibitor. In the European Union, Rinvoq has been approved for 3 indications: (1) It is used to treat moderate to severe rheumatoid arthritis (RA) that has insufficient or intolerance to one or more disease-modifying anti-rheumatic drugs (DMARD). ) Adult patients; (2) For the treatment of active psoriatic arthritis (PsA) adult patients with insufficient or intolerance to one or more DMARDs; (3) For the treatment of activities that do not respond to conventional therapies Adult patients with ankylosing spondylitis (AS). Among these indications, the approved dose of Rinvoq is 15 mg.
In the United States, Rinvoq is only approved for the treatment of moderate to severely active rheumatoid arthritis (RA) adult patients with insufficient or intolerance to methotrexate (MTX). The approved dose for this indication is 15 mg . Currently, Rinvoq’s supplementary application for the treatment of PsA and AS is under review by the US FDA.
In the European Union and the United States, Rinvoq’s application for new indications for the treatment of moderate to severe atopic dermatitis (AD) is also undergoing regulatory review. Currently, Rinvoq’s Phase 3 clinical studies for the treatment of UC, RA, PsA, AD, axial spondyloarthritis (axSpA), Crohn’s disease (CD), and giant cell arteritis (GCA) are underway.
The industry is very optimistic about Rinvoq’s business prospects. UBS analysts previously predicted that Rinvoq and AbbVie’s other monoclonal antibody anti-inflammatory drug Skyrizi will have a peak sales of 11 billion U.S. dollars. These two new products will be able to make up for the loss of sales caused by the impact of biosimilars on AbbVie’s flagship product Humira (Humira, adalimumab).
Humira is the world’s first approved anti-tumor necrosis factor alpha (TNF-α) drug and the world’s best-selling anti-inflammatory drug. Its global sales in 2020 are close to 20 billion U.S. dollars (19.832 billion U.S. dollars). In the European Union, a number of adalimumab biosimilars have been on the market. In the US market, Humira will be hit by biosimilars in 2023
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



