CAR-T: Conquer Lymphoma Lancer king and childhood brain tumors
c
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
CAR-T: Conquer Lymphoma Lancer king and childhood brain tumors
CAR-T: Conquer Lymphoma Lancer king and childhood brain tumors. CCAR-T therapy is newly upgraded! Finding an effective target and conquering lymphoma, cancer king, and childhood brain tumors is not far!
Blood tumor! Solid tumor! The new CAR-T therapy can’t let go.
Speaking of the hottest cancer therapy in recent years, the chimeric antigen receptor T cell (CAR-T) in immunotherapy is definitely one of the focuses.
According to experts from Cancer Free Homeland, CAR-T cells are called chimeric antigen receptor T cells. The basic principle of CAR-T cell therapy is to extract the patient’s own key immune cells—T cells, and embed it with a chimeric antigen receptor (CAR) that can activate T cells while recognizing tumor cell surface antigens. Cells kill tumor cells.
For example, if T cells are an “ordinary army”, then CAR-T cells are a “special force” with GPS, which can accurately locate and destroy the “enemy army”, that is, tumor cells. , To achieve targeted therapy for patients. At present, the technology has reached the domestic leading and international first-class level.
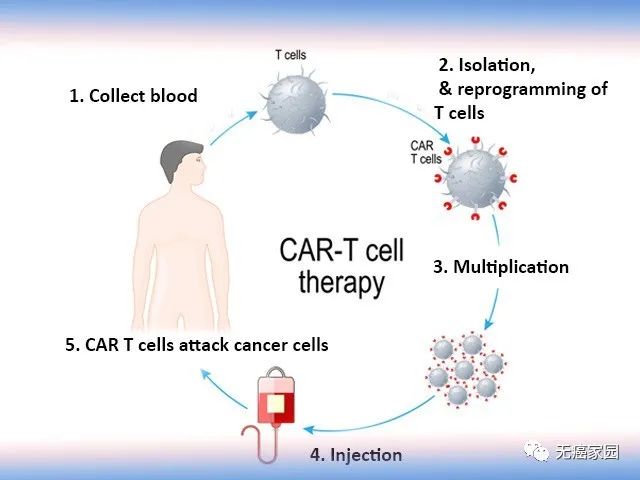
Today, the editor will sort out and summarize the “out of circle” research on CAR-T cell therapy in the treatment of hematological tumors and solid tumors in the past 3 months for your reference!
The total effective rate is 100%, and the anti-lymphoma effect of the new CAR-T therapy is amazing!
On January 27, the internationally renowned journal “Science Translation Medicine” published the latest research results of Yang Xuanming, Dean of the School of Life Science and Technology of Shanghai Jiaotong University. This study found for the first time that the antigen is independent of OX40 signal, which provides a new strategy for improving the anti-tumor activity of CAR-T cells and breaking the challenges of solid tumor treatment.

The researchers added a full-length costimulatory receptor to the CD3ζCAR (20BBZ CAR) vector with the costimulatory molecule 4-1BB, which is currently the most widely used clinically, and constructed a new type of CAR-T cell targeting CD20.
By comparing 12 costimulatory receptors, the researchers found that 20BBZ-OX40 CAR-T cells containing the OX40 costimulatory receptor have better expansion and anti-tumor capabilities.
As we all know, CAR-T immune cell therapy has achieved remarkable curative effects in the treatment of patients with malignant hematological tumors, and has brought great success in the research field of adoptive cell therapy. Compared with the traditional 20BBZ-CAR-T cells, the survival time of leukemia tumor-bearing mice is prolonged after treatment with the new CAR-T cell therapy (20BBZ-OX40 CAR-T).
In the subsequent preliminary clinical trials of patients with refractory, relapsed and metastatic B lymphoma, 5 patients were recruited, all of whom completed 20BBZ-OX40 CAR-T cell therapy.
The results are amazing! Of the 5 patients, 2 had complete remission and 3 had partial remission. The total effective rate was 100%.
Moreover, this new type of CAR-T cell has a very strong expansion ability in patients. In one patient, its CAR-T cells accounted for more than 80% of T cells in the peak value of proliferation. At the same time CAR-T has good safety!
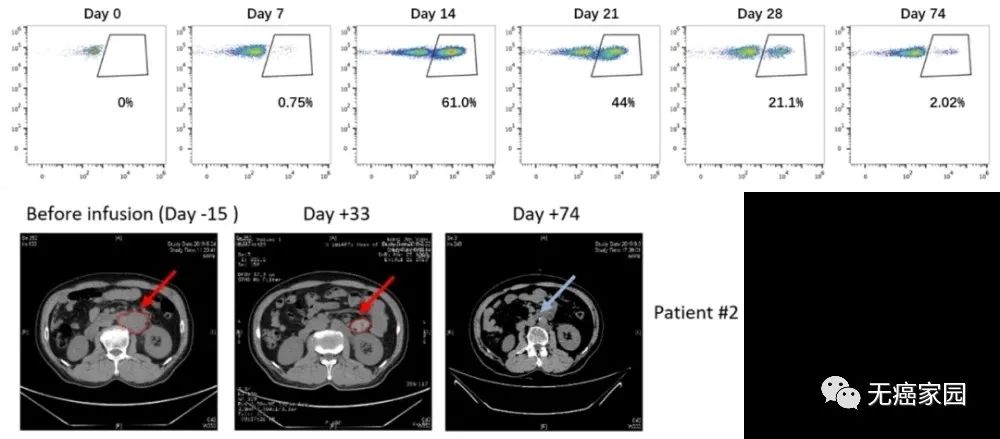
Amplification of the new CAR-T in patient 2 (top) and anti-tumor ability (bottom)
CAR-T therapy is expected to use new targets for pancreatic cancer to successfully fight cancer!
The cancer king dispute has always been an enduring topic. When it comes to the “cancer king”, pancreatic cancer deserves it. When it is diagnosed, only 20% of people are in the early or middle stage, which means that about 80% of patients have no chance of treatment, and the 5-year survival rate is only 10%. It is predicted that the mortality rate of pancreatic cancer in 2030 will be second only to Lung cancer.
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer, accounting for about 95% of pancreatic malignancies, and has the lowest survival rate among all common pancreatic cancers. In the UK, the 5-year survival rate for pancreatic ductal adenocarcinoma is only 7 %.
On January 21, an article published in “Clinical Cancer Research” found a new target suitable for the treatment of pancreatic ductal adenocarcinoma, and it is expected to use CAR-T cell therapy to combat pancreatic cancer.

This new target suitable for the treatment of pancreatic ductal adenocarcinoma is a protein called CEACAM7, which is abundantly expressed in pancreatic ductal adenocarcinoma, while the expression of this protein is hardly found in tonsils, lungs, liver, and prostate. This means that it may be an important target for CAR-T cell targeted therapy of pancreatic ductal adenocarcinoma.
In the study of CAR-T cells in preclinical models, it was found that it can effectively kill CEACAM7-expressing cells in pancreatic ductal adenocarcinoma culture, thereby effectively curbing the development of pancreatic cancer and successfully destroying cancer cells.
The most common brain tumor in children: neuroblastoma, ushering in new progress in CAR-T therapy!
It is mentioned that many parents of children with neuroblastoma suffer from headaches. This is a tumor that appears in immature nerve cells and accounts for more than 10% of all childhood cancer deaths. Almost 90% of neuroblastoma is at the age of 5 years. Found in the following children.
At the same time, it is also the third most common type of cancer in children, and it is rare in people over 10 years of age.
According to statistics, the 5-year survival rate of neuroblastoma is 80%. However, the survival rate of children depends on many factors, especially the risk group of tumors.
For children with low-risk neuroblastoma, the 5-year survival rate is higher than 95%. For children with moderate-risk neuroblastoma, the 5-year survival rate is between 90% and 95%. For high-risk neuroblastoma, the 5-year survival rate is about 40% to 50%.
This shows that neuroblastoma is so harmful and extremely dangerous!
In recent years, CAR-T cell therapy has opened up a new world in the field of advanced relapse and refractory B-cell tumors, and it has also allowed people to see the magic of this therapy.
Therefore, for the corresponding target of neuroblastoma, researchers have also developed the corresponding GD2-CAR-T cell therapy for the treatment of neuroblastoma.

The results showed that 12 children with relapsed/refractory neuroblastoma were generally well tolerated and had no off-target effects. Although they did not achieve an objective clinical response, they have seen practical results in some patients. The therapeutic effect.
Blood tumor! Solid tumor! The new CAR-T therapy never let go
In addition to the aforementioned breakthrough clinical studies of CAR-T therapy in hematological tumors and solid tumors, relevant CAR-T clinical studies are currently being actively carried out in China.
The emergence of CAR-T cell immunotherapy has given tumor patients hope. Cellular immunotherapy represented by CAR-T therapy will be the hottest field in the global biopharmaceutical industry in the next 5 to 10 years. I believe that in the near future, more and more clinical trial data will continue to emerge. CAR-T therapy will not only shine in the field of hematological tumors, but also increasingly demonstrate its hidden strength in solid tumors!
(source:internet, reference only)
Disclaimer of medicaltrend.org



