The results of the first clinical trials of CAR-T in the treatment of solid tumors
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The results of the first clinical trials of CAR-T in the treatment of solid tumors
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The latest paper of the father of CAR-T: The results of the first clinical trials of CAR-T in the treatment of solid tumors.
CAR-T , chimeric antigen receptor T cell immunotherapy. To put it simply, CAR-T is to transform the patient’s immune T cells in vitro through biotechnology to make them recognize antigens on the surface of tumor cells, and then infuse these cells back into the patient’s body to achieve the therapeutic effect of identifying and killing cancer cells.
In 2017, the FDA first approved the listing of CAR-T therapy for the treatment of blood cancers such as leukemia and lymphoma. The successful application of CAR-T therapy has rekindled hope for many desperate people waiting for bone marrow matching.
It also marks the arrival of the era of cell therapy. Up to now, the FDA has approved 5 CAR-T therapies for marketing, and some countries has also approved two CAR-T therapies in 2021.
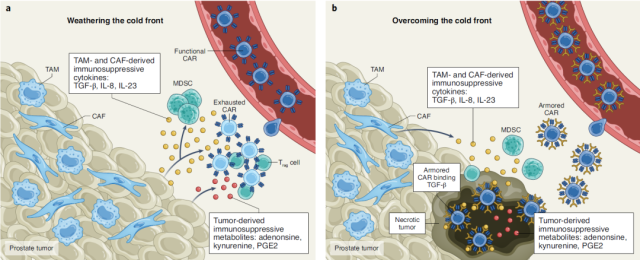
However, CAR-T has not yet achieved a breakthrough in solid tumors. One of the challenges is the existence of an immunosuppressive tumor microenvironment (TME) in solid tumors , which is characterized by the high expression of various inhibitory factors, such as TGF-β .
Prostate cancer is the second most common malignant tumor in men. In 2020, there will be 1.41 million new prostate cancer patients worldwide, second only to lung cancer in men.
For prostate cancer, the current main treatment isantiandrogen therapy, however, many patients will progress to castration-resistant prostate cancer (CRPC)and metastasize.
There are currently no effective treatments for trend-resistant prostate cancer, and patients have poor clinical outcomes, necessitating the development of new therapies.
On March 21, 2022, Carl June et al . of the University of Pennsylvania published the title: PSMA-targeting TGFβ-insensitive armored CAR-T cells in metastatic castration-resistant prostate cancer: a phase 1 trial in Nature Medicine , a top international medical journal research paper.
The research team developed a CAR-T therapy targeting prostate – specific membrane antigen (PSMA) , and further optimized the CAR-T cells to overexpress dominant-negative TGFβRII (TGFβRDN) , which can block TGF-β signaling to enhance antitumor immunity in patients .
Data from this phase 1 clinical trial demonstrate that anti-TGF-beta CAR-T cell therapy for solid tumors is feasible and generally safe . Multi-pronged approaches targeting the tumor microenvironment of solid tumors should be investigated in the future to further improve treatment outcomes.

The development of cancer is usually caused by genetic abnormalities, which may lead to the expression of some proteins in the tumor that are completely absent in normal tissues.
This protein is also called neoantigens (neoantigens) . , and low expression in normal tissues, this protein is called tumor associated antigen (tumor associated antigen) .
Prostate-specific membrane antigen (PSMA) is highly expressed in metastatic castration-resistant prostate cancer (mCRPC) and represents a promising tumor-associated antigen for immunotherapy.
However, applying CAR-T therapy to the treatment of mCRPC still faces a major challenge— the immunosuppressive tumor microenvironment . In particular, mCRPC has a markedly high expression of TGF-β in the tumor microenvironment, which severely limits the therapeutic potential of engineered T cells.
Previous preclinical studies have shown that overexpression of a dominant-negative effector TGFβRII (TGFβRDN) in CAR-T cells or knockdown of TGFBR2 via CRISPR-Cas9 can inhibit TGF-β signaling, thereby significantly enhancing the effect of CAR-T on tumors. inhibitory effect.
Therefore, the research team hopes to develop anti- TGF-β CAR-T cell therapy for the treatment of metastatic castration-resistant prostate cancer (mCRPC) .
The CAR-T therapy developed by the research team targets prostate-specific membrane antigen (PSMA) , and the CAR-T is optimized to express TGFβRDN , thereby enhancing TGF-β signaling by blocking TGF-β signaling. Antitumor immunity of patients.
The primary endpoint of this phase 1 clinical trial is the safety and feasibility of the treatment, and secondary endpoints include assessing the distribution, biological activity and disease response of CAR-T cells.
The research team also performed a comprehensive analysis of the tumor microenvironment to identify determinants of antitumor efficacy and drug resistance.
Eighteen patients with metastatic castration-resistant prostate cancer (mCRPC) participated in this phase 1 clinical trial, 13 of whom received treatment at four dose levels. Five of these 13 had a grade ≥2 cytokine storm, and four had a ≥30% drop in prostate-specific antigen (PSA) .
One person developed massive clonal CAR-T cell expansion, a >98% PSA drop, and later died of sepsis-induced organ failure.
Overall, first, considerable efficacy was observed in at least 1 patient, and additional therapeutic activity was observed in the cold tumor microenvironment of an additional 3 patients. Second, the assay showed that anti-TGF – β CAR -T cells reduced TGF-β signaling in tumors; In-depth understanding of security.
Immune checkpoint therapy has made progress and breakthroughs in a variety of malignancies, arguably changing the cancer treatment landscape, however, prostate cancer patients have not been able to benefit from it. The results of this clinical trial let us see the hope of immunotherapy for prostate cancer.
It should be pointed out that, as a tumor associated antigen , PSMA is not only highly expressed in prostate cancer cells, but also has a small amount of expression in normal prostate tissue, peripheral blood, central nervous system, small intestine and other tissues.
PSMA is expressed in normal tissues. Widespread distribution may lead to cytokine storms during therapy, which is a concern.
In addition to PSMA, there are two ongoing clinical trials targeting prostate stem cell antigen (PSCA) (clinical trial numbers: NCT03873805, NCT02744287) , and PSCA is also expressed in small amounts in various normal tissues.
Reference :
https://www.nature.com/articles/s41591-022-01726-1
https://www.nature.com/articles/s41591-022-01742-1
The results of the first clinical trials of CAR-T in the treatment of solid tumors.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



