Trastuzumab Deruxtecan: Big change in treatment of HER-2+ solid tumors
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Trastuzumab Deruxtecan: Big change in treatment of HER-2+ solid tumors
Trastuzumab Deruxtecan: Big change in treatment of HER-2+ solid tumors. HER2 targeted therapy significantly improved the prognosis of HER2-positive breast cancer patients and gastric cancer patients.
At present, several anti-HER2 targeted drugs have been approved for the treatment of breast cancer: trastuzumab and pertuzumab, both of which are anti-HER2 human monoclonal antibodies, and combined chemotherapy has been approved as the first-line treatment for metastatic breast cancer.
In addition, there are ADC drugs trastuzumab emtansine (T-DM1) and HER2 kinase inhibitor lapatinib for further treatment. HER2 overexpression and mutation are also pathogenic factors for non-small cell lung cancer (NSCLC) and colorectal cancer. However, to date, there is no approved HER2 targeted therapy for these indications.
Trastuzumab deruxtecan (T-DXd) (DS-8201; ENHERTU®) is a new HER2 targeting ADC jointly developed by Daiichi Sankyo and AstraZeneca. T-DXd has shown significant anti-tumor activity in the treatment of refractory HER2+ metastatic breast cancer patients. Therefore, T-DXd was approved in 2019.
T-DXd has shown potential anti-tumor activity in several HER2-expressing solid tumors, including HER2-low-expressing breast cancer, HER2-positive gastric cancer, HER2-expressing colorectal cancer, and HER2-expressing or mutated NSCLC. At present, a number of clinical trials are underway to explore the activity of T-DXd in tumors that widely express HER2. The existing preliminary results show that T-DXd has the potential to change the treatment model of HER2+ tumors.
Mechanism of action of T-DXd
HER2 belongs to the four tyrosine kinases of the human epidermal growth factor receptor (HER) family, including EGFR (HER1, erbB1), HER2 (erbB2, HER2/neu), HER3 (erbB3) and HER4 (erbB4). These receptors regulate key cellular processes, including proliferation, movement, and survival. HER2 overexpression has a high transformation potential, and the amplification of the HER2 locus is an early event of breast cancer carcinogenesis.
T-DXd is an antibody-conjugated drug, consisting of anti-HER2 humanized monoclonal antibody (MAAL-9001) and Isartecan derivative MAAA-1181a (DXd). The partial amino acid sequence of the antibody is combined with trastuzumab. Similarly, DXd is a DNA topoisomerase I inhibitor. Compared with the active metabolite SN-38 of irinotecan, its inhibitory potency is 10 times higher.
After binding to HER2, T-DXd disrupts the HER2 signal and mediates antibody-dependent cytotoxicity. In addition, after binding, T-DXd undergoes endocytosis and is lysed in the cell, leading to the release of DXd, which in turn leads to DNA damage and cell apoptosis.
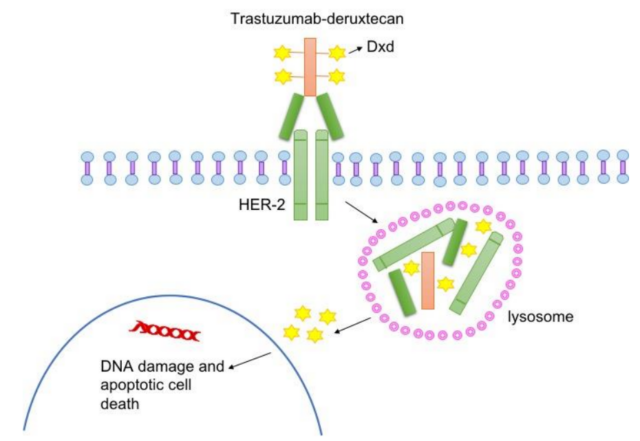
T-DXd is specially designed to improve the characteristics of other anti-HER2 ADCs. Trastuzumab can regulate the expression of topoisomerase I in extracellular vesicles released by HER2-positive cancer cells. The combination of topoisomerase inhibitor DXd and trastuzumab may have the potential to enhance anti-tumor effects effect.
Compared with TDM-1, T-DXd has a higher drug-to-antibody ratio (DAR, about 8:3-4), and is more effectively delivered to tumor cells expressing HER2. The half-life of its cytotoxic payload is short, thus increasing the cytotoxic effect, but minimizing systemic exposure and limiting off-target toxicity to normal cells. In addition, the payload has a high membrane permeability, which allows cytotoxic effects on tumor cells close to the target cell regardless of their HER2 expression level. This cytotoxic bystander effect has been confirmed in vitro and in vivo.
Clinical study of T-DXd
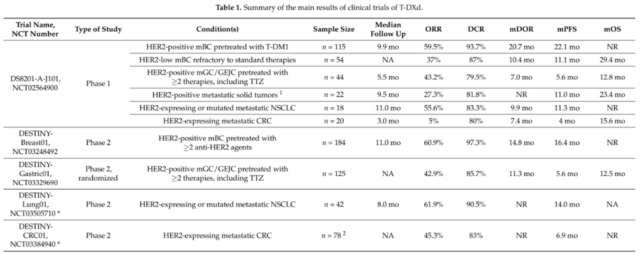
In a dose-escalation phase 1 clinical trial (NCT02564900), the safety, tolerability and efficacy of T-DXd in patients with advanced HER2-expressing breast and gastroesophageal junction tumors were evaluated. In the first part of this trial, 24 patients were included in the study and received T-DXd from 0.8 to 8.0 mg/kg every 3 weeks. In this small, refractory patient population, T-DXd shows good anti-tumor activity with a disease control rate (DCR) of 91%, even in tumors with low HER2 expression.
In the dose expansion part of this study, 60 patients received a dose of 6.4 mg/kg T-DXd ≥1 (colorectal cancer, n=20; NSCLC, n=18; others, n=22). In this highly heterogeneous population, the average progression-free survival (PFS) was 7.2 months (95%, CI, 4.8-11.1), and the objective response rate (ORR) was 28.3%. Interestingly, the median PFS in patients with non-small cell lung cancer reached 11.3 months (95% CI, 8.1 to 14.3), and the ORR was 72.7%. The overall T-DXd has acceptable safety.
Breast cancer
In the dose expansion part of the phase 1 clinical trial, the ORR for metastatic refractory breast cancer was 59.5% and the DCR was 93.7%. The median PFS was 22.1 months, and the median OS was not reached. In the same phase 1 trial, T-DXd also showed anti-tumor activity in HER2 low-expressing breast cancer patients that were ineffective to standard treatments (n=54): the confirmed ORR was 37.0%, and the median remission (DOR) was 10.4 Months. The median PFS was 11.1 months, and the median OS was 29.4 months (95% CI, 12.9-29.4).
In the phase 2 DESTINY-Breast01 trial (NCT03248492), patients with HER2-positive metastatic breast cancer were evaluated for T-DXd activity. These patients had received ≥2 anti-HER2 medications, including TDM-1. The test results showed that ORR was 60.9% and DCR was 97.3%. The median DOR was 14.8 months, and the median PFS duration was 16.4 months. The median OS was not reached, but the estimated OS was 93.9% at 6 months and 86.2% at 12 months.
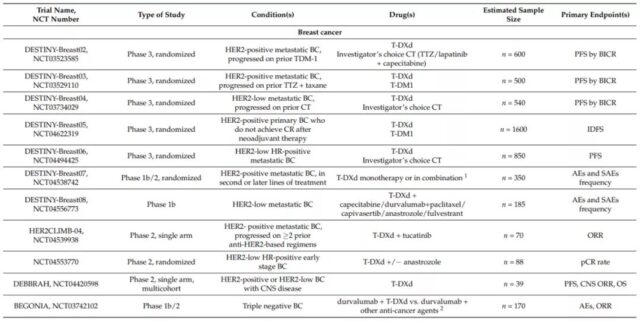
Based on this, on December 20, 2019, the FDA approved T-DXd for patients with unresectable or metastatic HER2-positive breast cancer. So far, there are still 5 phase 3 randomized trials on T-DXd in breast cancer patients, which are HER2 positive (DESTINY-Breast02, NCT03523585; DESTINY-Breast03, NCT03529110) and HER2 low expression (DESTINY-Breast04, NCT03734029; DESTINY -Breast06, NCT04494425) metastatic disease, and HER2-positive primary breast cancer patients did not achieve complete remission after neoadjuvant therapy (DESTINY-Breast05, NCT04622319). Several other phase 1b and phase 2 trials are evaluating the role of T-DXd in breast cancer central nervous system metastasis and triple-negative breast cancer patients.
Stomach cancer
In the extended phase I trial, 44 patients with HER2-positive gastric or gastroesophageal junction cancer received T-DXd treatment. After a median follow-up of 5.5 months, the ORR was 43.2%, 79.5% of the patients reached DCR, and the median DOR was 7.0. Months, the median PFS was 5.6 months, and the median OS was 12.8 months. In a post-hoc subgroup analysis of 24 patients who had received irinotecan, the ORR was 41.7% and the DCR was 79.2%.
The randomized phase 2 trial DESTINY-Gastric01 (NCT03329690) evaluated T-DXd in patients with HER2-positive advanced gastric or gastroesophageal junction adenocarcinoma. These patients had received at least two treatments, including trastuzumab. The results of the trial were announced at the 2020 ASCO Annual Meeting: Compared with the chemotherapy control group, ORR was 42.9% vs 12.5%, DCR was 85.7% vs 62.5%; median DOR was 11.3 vs 3.9 months; median PFS was 5.6 Month vs 3.5 months. T-DXd significantly prolonged OS, with a median OS of 12.5 vs 8.4 months, and a 12-month OS of 52.1% and 28.9%.
The efficacy of T-DXd was also evaluated in two exploratory cohorts of HER2 low expression gastric cancer, including IHC2+/ISH-patients (cohort 1, n=20) and IHC 1+ (cohort 2, n=24). In cohort 1, ORR was 26.3% and DCR was 89.5%. The median PFS was 4.4 months, the median OS was 7.8 months, and the 12-month OS accounted for 40.0%. In cohort 2, ORR was 9.5% and DCR was 71.4%. The median PFS was 2.8 months, the median OS was 8.5 months, and the 12-month OS accounted for 25.7%.
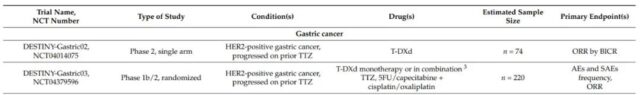
On January 15, 2021, the FDA approved T-DXd for the treatment of adult patients with locally advanced or metastatic HER2-positive gastric cancer or gastroesophageal junction adenocarcinoma. These patients have previously received trastuzumab-based Treatment programs. Currently, there are two phase 2 clinical trials in progress in HER2-positive gastric cancer patients, including the evaluation of T-DXd as monotherapy (DESTINY-Gastric02, NCT04014075) or combination chemotherapy and trastuzumab (DESTINY-Gastric03, NCT04379596) ).
Non-small cell lung cancer
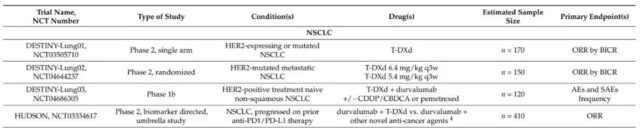
The DESTINY-Lung01 (NCT03505710) phase II clinical trial evaluated T-DXd in non-squamous NSCLC patients with HER2 overexpression or HER2 activating mutations. Preliminary data from this trial confirmed that T-DXd has a high ORR and a durable response in patients with HER2 mutations (n=42). At a median follow-up time of 8.0 months, the ORR was 61.9%, the median DOR was not reached, and the DCR was 90.5%. The estimated median PFS was 14.0 months (95% CI, 6.4-14.0 months). It is worth noting that 45.2% of patients have central nervous system metastases.
At present, there are four phase 2 studies and one phase 1 study for HER2-positive or mutant NSCLC patients: DESTINY-Lung01 (NCT03505710) and DESTINY-Lung02 (NCT04644237) evaluating T-DXd monotherapy, DESTINY-Lung03 ( NCT04686305) and the HUDSON trial (NCT03334617) evaluate the combination of T-DXd with immunotherapy, chemotherapy, and new anticancer drugs.
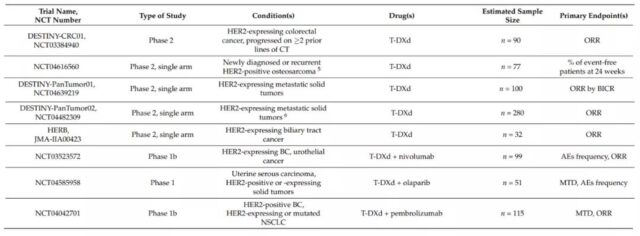
Colorectal cancer and others
DESTINY-CRC01 (NCT03384940) is a phase 2 clinical trial that evaluated T-DXd in patients with metastatic colorectal cancer that expresses HER2. In this study, patients were divided into 3 groups according to the expression level of HER2 (A: HER2 IHC 3+ or IHC 2+/ISH+; B: IHC 2+/ISH−; C: IHC 1+). Preliminary data show that in 78 patients with HER2-positive colorectal cancer (A n=53; B n=7; C n=18), T-DXd has a significant effect. The ORR of group A was 45.3% (95%CI, 31.6-59.6%); the median DOR was not reached (95%CI, 4.2 months-NE), and the DCR was 83.0% (95%CI, 70.2-91.9%); The median PFS was 6.9 months (95%CI, 4.1 months-NE); the median OS was not reached. No response was observed in group B or C.
At present, there are still a number of clinical trials evaluating T-DXd monotherapy and combination therapy in different cancers.
The safety of T-DXd
A comprehensive analysis of T-DXd was performed on 234 patients with HER2-positive breast cancer. The most common adverse events (≥20%) included: nausea, fatigue, vomiting, hair loss, constipation, decreased appetite, anemia, neutropenia, and diarrhea , Leukopenia, cough and thrombocytopenia. The most commonly reported grade 3-4 AEs (≥5%) included neutropenia (16%), anemia (7%), nausea (7%), fatigue (6%), and leukopenia (6%). The incidence of serious adverse events (SAE) was 20%, including interstitial lung disease (ILD), pneumonia, vomiting, nausea, cellulitis, hypokalemia, and intestinal obstruction.
9% of patients stopped treatment permanently due to SAEs, and most cases were ILD (6%). 33% and 18% of patients required dose interruption and dose reduction due to treatment-related toxicity. Fatal AEs occurred in 4.3% of patients. ILD was the most common cause (2.6%), followed by acute liver failure and acute kidney injury, deterioration of general health, pneumonia, and hemorrhagic shock (0.4% each).
Summary
T-DXd is a promising drug for the treatment of HER2-expressing solid tumors. In view of the remarkable success of T-DXd in the treatment of HER2-expressing tumors, it is currently undergoing multiple clinical trials at the same time, whether as a monotherapy or Combined treatment with new anti-cancer drugs.
Existing data indicate that T-DXd will become an important therapeutic weapon for the treatment of patients with HER2-positive breast cancer metastasis. The DESTINY-Breast03 trial is evaluating the head-to-head comparison of T-DXd and T-DM1 in HER2-positive breast cancer patients who have received trastuzumab combined with chemotherapy. Clinical trials are also evaluating T-DXd as a possible treatment strategy for neoadjuvant therapy and adjuvant therapy. T-DXd represents an emerging treatment strategy for patients with low HER2 tumors. T-DXd may become an effective alternative to chemotherapy for patients with low HER2 expression and hormone-positive breast cancer.
In HER2-positive gastric cancer patients, T-DXd has a good application prospect. So far, apart from trastuzumab, no other HER2-directed therapy has a significant effect on HER2-positive gastric cancer patients. A new treatment strategy for HER2-positive gastric cancer patients is approaching.
With the emergence of T-DXd, HER2 may become an important therapeutic target for patients with NSCLC and colorectal cancer. Although T-DM1 has achieved good response rates in different studies, ranging from 20% to 50%, it is still low compared to the response rate observed with T-DXd (ORR 61.9%). Even in patients with HER2-positive colorectal cancer, single-agent T-DXd showed an ORR of more than 50%, while the ORR of trastuzumab combined with lapatinib or pertuzumab was only 30%. In general, preliminary evidence indicates that T-DXd is actively changing the treatment model for HER2-positive tumors.
(source:internet, reference only)
Disclaimer of medicaltrend.org



