Progress of AKT allosteric inhibitor TAS-117 for advanced solid tumors
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Clinical research progress of AKT allosteric inhibitor TAS-117 for advanced solid tumors
Progress of AKT allosteric inhibitor TAS-117 for advanced solid tumors. PI3K/AKT/mTOR signaling pathway plays an important role in cell proliferation, cell cycle, apoptosis and tumor cell metabolism.
The dysregulation of this signaling pathway is associated with the occurrence of a variety of tumors. Therefore, the use of targeted drugs to inhibit the PI3K/AKT/mTOR pathway may be beneficial to the treatment of clinical patients with mutations related to this pathway.
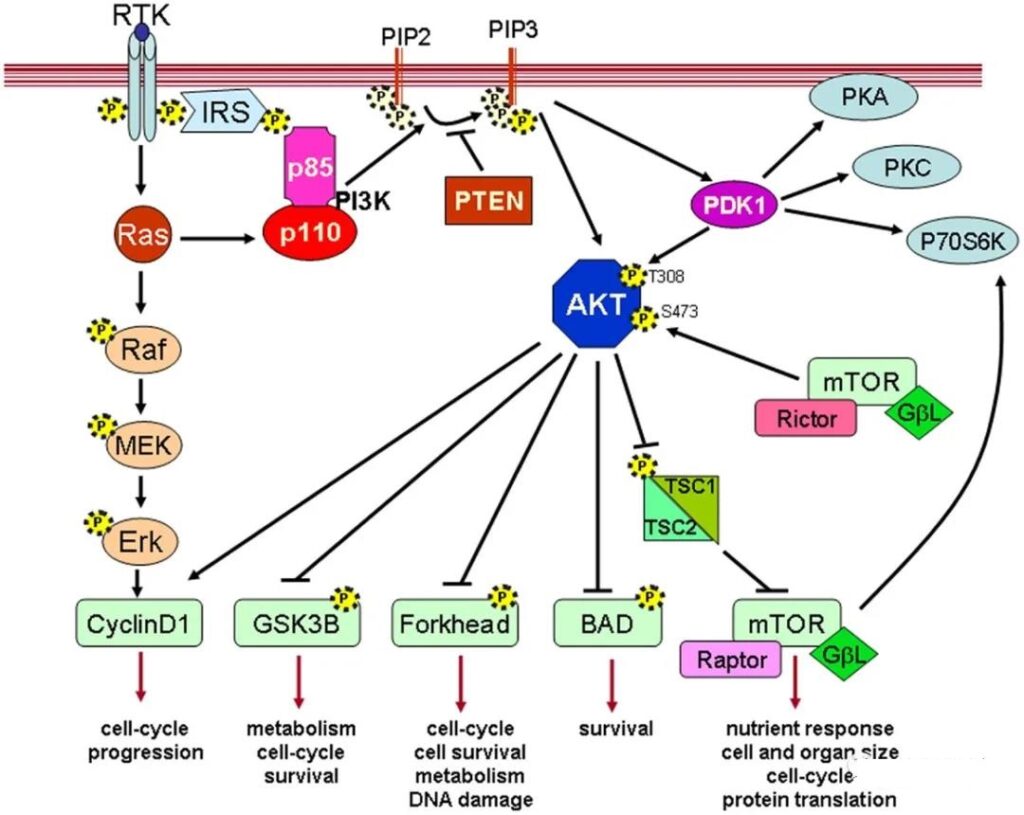
In the past few years, more than forty innovative compounds targeting the PI3K/AKT/mTOR pathway have appeared. However, only a few drugs have been approved by the FDA, such as mTOR inhibitors (temsirolimus and everolimus) and PI3K inhibitors (idelalisib, PI3K-δ specific inhibitors; copanlisib, PI3K inhibitors that are mainly active against PI3K-α and PI3K-δ; alpelisib, PI3K-α specific inhibitor).
Although approved by the FDA, mTOR allosteric inhibitors such as temsirolimus and everolimus have a low objective response rate when used as a single treatment. Except for copanlisib, PI3K pan-inhibitors exhibited intolerable toxicity due to their broad-spectrum molecular activity. Other drugs have failed due to poor efficacy, high toxicity, or lack of reliable predictive biomarkers.
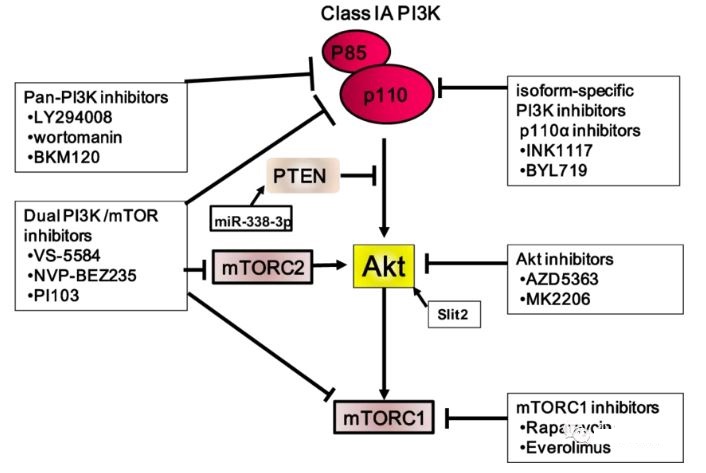
Some preclinical studies using cell lines and xenograft tumors have shown that targeting the downstream Akt pathway can reduce cell proliferation in a variety of tumor cell lines.
Akt activation leads to abnormalities in upstream regulators, including
(1) Upstream activation or gene amplification of receptor tyrosine kinases,
(2) Amplification or mutation of PI3K catalytic subunit α (PIK3CA) gene encoding PI3K p110α catalytic subunit,
(3) Gene silencing of the tumor suppressor gene PTEN, which negatively regulates the PI3K pathway.
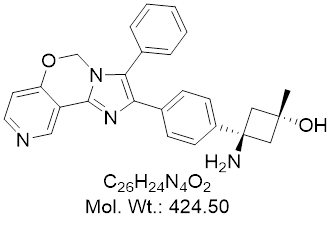
TAS-117 is a potent and selective oral Akt allosteric inhibitor, showing high affinity for all three subtypes (Akt1, 2, and 3). It effectively inhibits the proliferation of breast cancer, endometrial cancer, lung cancer and ovarian cancer cells in vitro. In addition, tumor cell lines containing Akt2 and HER2 gene amplification, PI3K mutation and PTEN deletion are more sensitive to TAS-117.
In a xenograft tumor model in nude mice, daily administration of TAS-117 exhibited a significant dose-dependent anti-tumor effect. In the Phase I clinical study of TAS-117, 60 evaluable patients with advanced solid tumors showed promising objective remission, especially patients with ovarian cancer.
In addition, TAS-117 has shown its safety in patients with all cancer types. Due to the dose-limiting toxicity of plaque papules, the recommended phase 2 dose (RP2Ds) is 16 mg per day for patients with gastrointestinal (GI) tumors and 24 mg per day for patients with non-gastrointestinal tumors.
It is taken for 4 days or 3 days off. Therefore, as part of the K-BASKET trial, the researchers conducted a phase II clinical study of TAS-117 in patients with advanced solid tumors with abnormal PI3K/Akt genes.
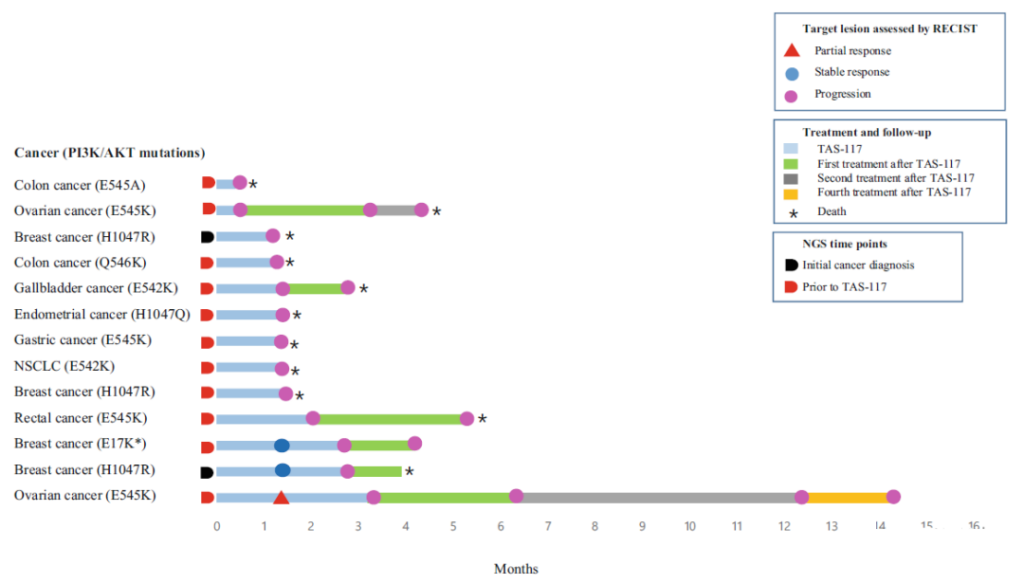
From November 21, 2017 to June 27, 2019, a total of 13 patients with advanced solid tumors with PI3K/AKT gene abnormalities were screened for this clinical study. Among them, 8 patients had non-gastrointestinal tumors (GI) (breast cancer, ovarian cancer, endometrial cancer, and non-small cell lung cancer), and 5 patients had GI (colon cancer, rectal cancer, gastric cancer, and gallbladder cancer). Ten patients received TAS-117 treatment after ≥4 lines of treatment. Twelve patients contained PIK3 catalytic subunit α (PIK3CA) mutations (E542K, E545A, E545K, H1047R and Q546K); one of them contained Akt1^E17K mutation.
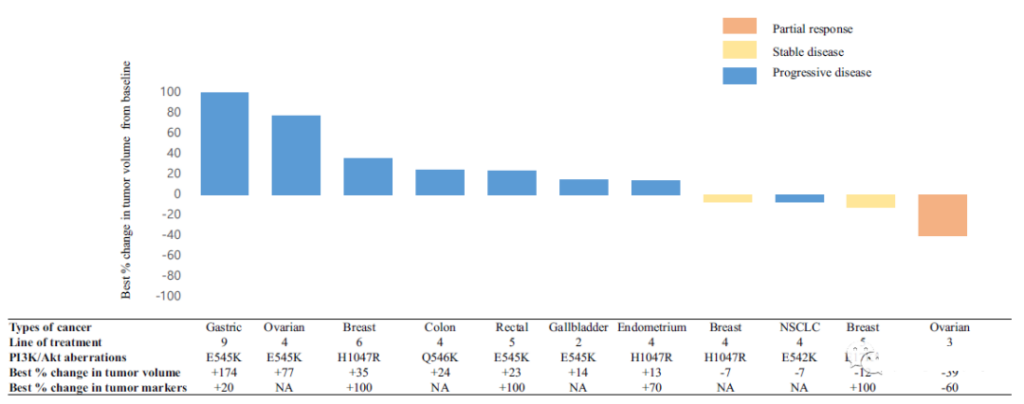
The average treatment time was 1.4 months, and the average treatment cycle was 2 rounds.
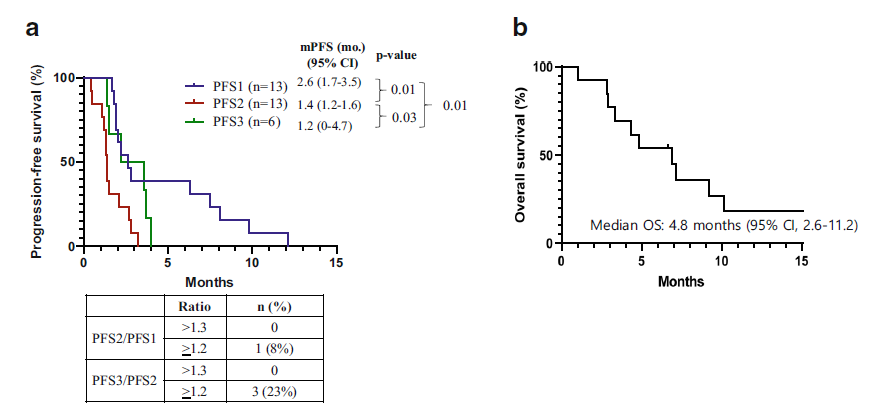
The evaluation of its anti-tumor efficacy is as follows, the overall response rate (ORR) is 8%, and the disease control rate (DCR) is 23%; the average progression-free survival (PFS) and overall survival (OS) are 1.4 months and 4.8, respectively month.
Eleven patients (85%) had ≥ Grade 1 treatment-related adverse reactions, including hyperglycemia (n=4, 36%), skin rash (n=4, 36%), and anorexia (n=4, 36%) , Nausea (n=2, 15%) and diarrhea (n=2, 15%). Adverse reactions caused by grade 3-4 treatment include anorexia (grade 3, 8%) and hyperglycemia (grade 3, 8%; grade 4, 8%).
Comparing the clinical phase I results of TAS-117, 20 of 62 patients with advanced solid tumors had ovarian clear cell carcinoma, of which 5 (25%) had tumors shrunk by more than 30%, and 3 (15%) had Continuous efficacy. Similarly, in this phase 2 study, one-quarter of ovarian cancer patients showed partial remission, but the remission was not lasting, and disease progression occurred after 18 weeks.
Overall, the short progression-free survival (PFS) and the insignificant progression-free survival ratio reflect the only weak effect of TAS-117 as a single-dose treatment. The Independent Data Monitoring Committee recommends that this study be terminated as soon as possible and that future combination therapies should be developed as an earlier treatment plan. The main reasons are as follows:
(1) Severely treated patients with different cancer types that are ineffective to standard treatments are resistant to TAS- 117 lacks a lasting response,
(2) the frequency of PI3K/Akt gene mutation is low.
The low efficacy of TAS-117 may be caused by the following factors. (1) Inhibition of Akt alone is not sufficient to target PI3K/Akt/mTOR pathways because of complex changes; (2) There is currently no standardized and reliable predictive biomarker to select acceptable targets for PI3K/Akt /mTOR pathway drug therapy; (3) The type of tumor depends on the degree of tumorigenicity, and the duration and depth of response to drugs targeting the PI3K/Akt/mTOR pathway are different.
In summary, the Akt allosteric inhibitor TAS-117 has shown limited anti-tumor activity and controllable toxicity in a variety of patients with advanced solid tumors. Its clinical efficacy has been observed in patients with ovarian cancer (PIK3CA E545K mutation) and breast cancer (PIK3CA H1047R and Akt1 E17K mutation).
By combining chemotherapy, targeted therapy and immunotherapy, including TAS-117 into the early treatment line for breast cancer and ovarian cancer patients with PIK3CA E545K, H1047R and Akt1 E17K mutations can overcome the new drug resistance of TAS-117.
(source:internet, reference only)
Disclaimer of medicaltrend.org



