New Potential Target in Cancer Treatment: SRPKs
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New Potential Target in Cancer Treatment: SRPKs
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
New Potential Target in Cancer Treatment: SRPKs
Kinases regulate various cellular processes, including proliferation, differentiation, apoptosis, movement, transcription, and translation, by adding phosphate groups to target proteins. These phosphorylation events act as molecular switches, modulating or controlling the biological functions of target proteins.
Serine-arginine (SR) protein kinases (SRPKs) are a subfamily of serine-threonine kinases, including SRPK1, SRPK2, and SRPK3. SRPKs phosphorylate multiple serine residues within the C-terminal arginine-serine-rich domain of splicing factors involved in the regulation of the serine-arginine (RS) domain.
Dysregulation of SRPKs is associated with various types of cancer.
Overexpression of SRPKs is observed in breast cancer, prostate cancer, lung cancer, colorectal cancer, and pancreatic cancer, promoting the splicing of various oncogenic isoforms.
Inhibition of SRPKs has been shown to reduce the growth and migration of cancer cells, suggesting their potential as promising targets for cancer treatment.
Lung Cancer
The SRPK pathway is implicated in lung cancer, with dysregulation of SRPKs observed in both non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC).
Increased expression of SRPK1 promotes tumor growth and migration. Conversely, reducing SRPK1 expression inhibits tumor migration, metastasis, and growth. SRPK1 activates a protein complex called β-catenin/T-cell factor (TCF), and reducing SRPK1 expression decreases the activity of genes controlled by this complex (Figure 1A).
In Wilms’ tumor cells, Wilms’ tumor suppressor 1 (WT1) inhibits SRPK1. SRPK1, in turn, affects the activity of its substrate, the prototypical SR protein SRSF1, and alters the splicing of the key vascular endothelial growth factor (VEGF). In both normal and tumor blood vessels, the expression of proteins WT1, SRPK1, SRSF1, and the vascular growth factor VEGF164A subtype is higher than in normal vessels. SRSF1 protein is present in the vascular nuclei of various tumor types but absent in healthy tissues. Deleting vascular-specific WT1 protein reduces the expression of WT1, SRPK1, and SRSF1 proteins, leading to a shift towards the expression of the anti-angiogenic VEGF120 subtype (Figure 1B).
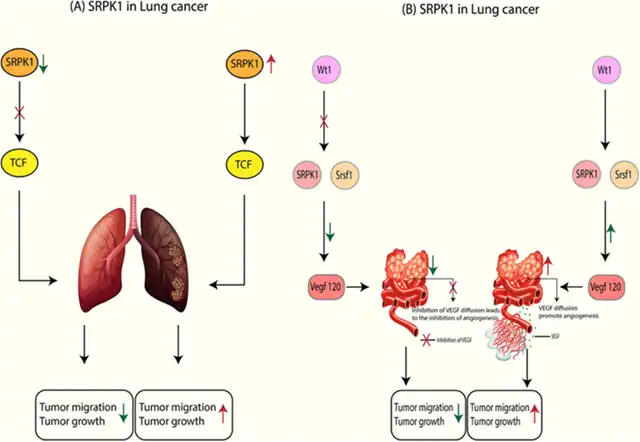
Figure 1
In non-small cell lung cancer (NSCLC), SRPK2 activates the splicing factor SC35, which, in turn, activates cell cycle-related genes, promoting tumor progression (Figure 2).
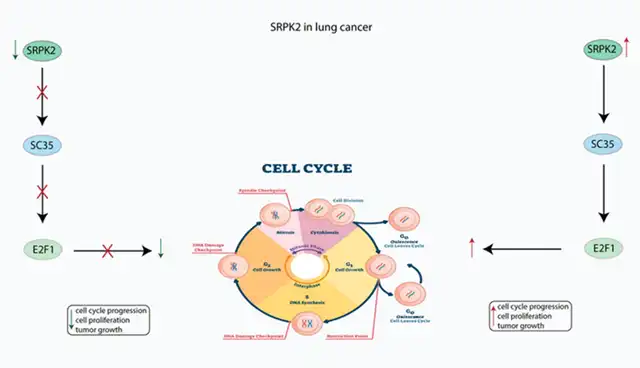
Figure 2
Breast Cancer
Studies indicate that SRPKs are upregulated in breast cancer (BC) and associated with invasive tumors. In preclinical models, inhibiting SRPKs reduces the growth and survival of BC cells.
LIM kinase 2 (LIMK2) is a serine-specific kinase crucial for the distant metastasis of triple-negative breast cancer (TNBC). It is activated through phosphorylation by SRPK1, enhancing its kinase activity.
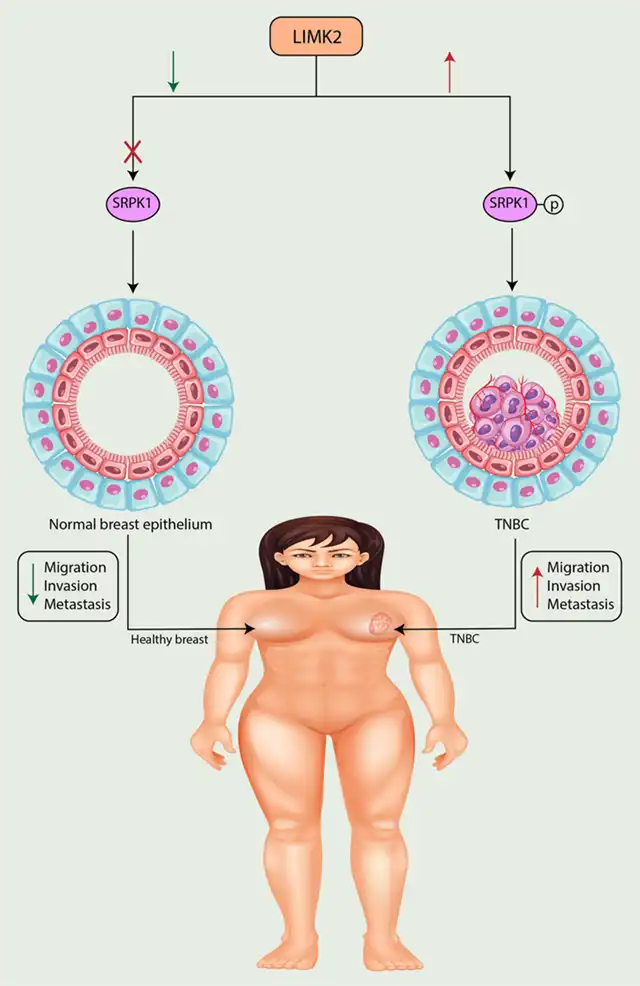
Figure 3
The expression of fatty acid synthase (FASN) is associated with a more invasive phenotype of breast cancer and is regulated downstream of the receptor tyrosine kinase (RTK) signaling pathway. Recently, post-transcriptional regulation of fatty acid synthesis transcripts has been shown to be mediated by SRPK2 downstream. SRPK2 acts on phosphorylating splicing factors rich in serine-arginine (SRSF), leading to RNA binding and various RNA regulatory processes.
The latest findings establish a potential IGF-1-mTORC1-SRPK2-FASN axis in cancer, which could be a potential therapeutic target for cancers overexpressing FASN and IGF-1R pathway components.
When insulin-like growth factor 1 (IGF-1) is present, both FASN mRNA and protein expression increase. However, this increase decreases when the mTORC1 pathway is inhibited. Additionally, the inhibition of mTORC1 by IGF-1 leads to a decrease in p-SRPK2 levels. Moreover, when SRPK2 is specifically knocked down or inhibited, the stability of FASN mRNA and protein decreases, especially in triple-negative or HER2+ breast cancer cell lines.
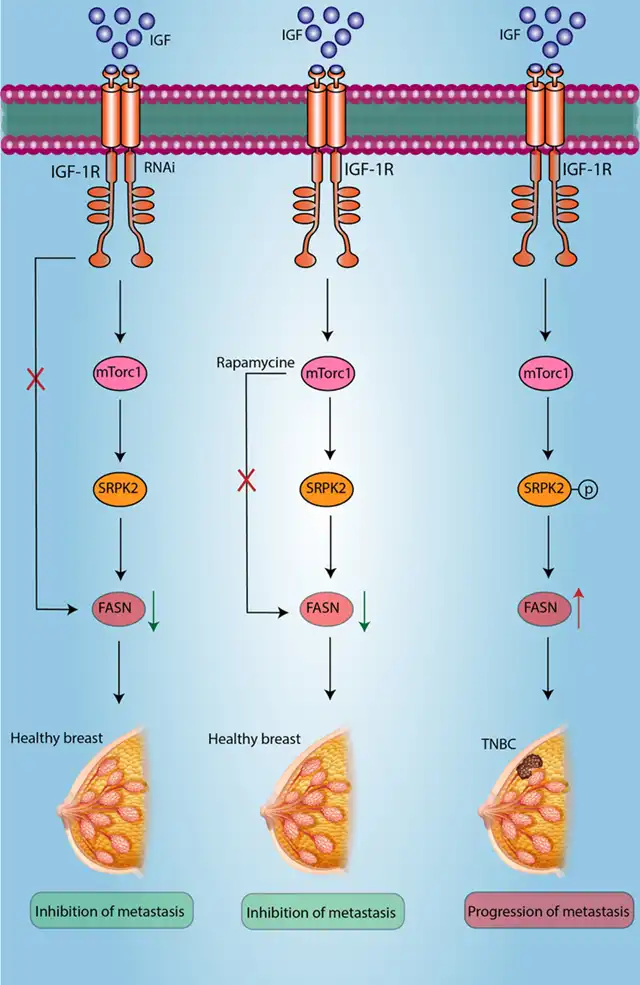
Figure 4
Colorectal Cancer
In colorectal cancer, dysregulation of SRPKs is associated with poor prognosis. In preclinical studies, inhibition of SRPKs can reduce the growth and survival of colorectal cancer cells, suggesting that SRPKs may be useful therapeutic targets in colorectal cancer treatment.
Human MAP kinase-interacting serine/threonine kinase 2 (MKNK2) has two different messenger RNA subtypes, translating into two distinct protein isoforms. MKNK2a promotes tumor regression, while MKNK2b promotes cancer growth.
In colorectal adenocarcinoma cells, however, SRPK1 and SRPK2 are overexpressed, PP1α is inactive, leading to the overphosphorylation of SRSF1. This phosphorylation causes the transport of SRSF1 to the cell nucleus in a TNPO3-dependent manner, where it directly binds to MKNK2 pre-mRNA, increasing the quantity of MKNK2b isoform and decreasing the proportion of MKNK2a.
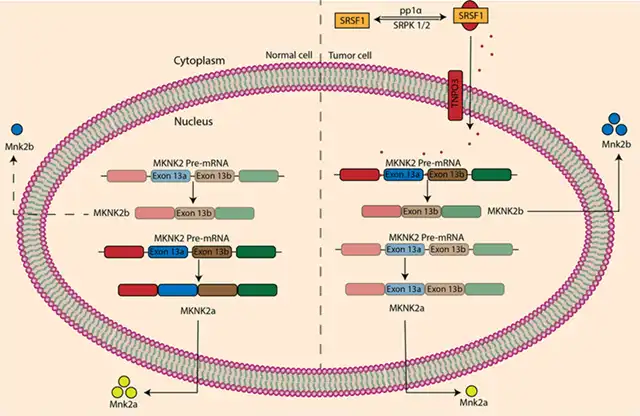
Figure 5
SRPKs constitute an enzyme family that regulates gene expression and splicing. Dysregulation of SRPKs is associated with the development and progression of various cancers, and targeting SRPKs may have therapeutic potential in cancer treatment. However, more research is needed to fully understand the role of SRPKs in cancer and identify the most effective targeted therapies.
Future research on SRPKs may include:
(A) Mechanisms of action in cancer cells, identifying the most important specific SRPKs in cancer, and developing specific drugs or other therapies targeting SRPKs.
(B) Development of small molecule inhibitors for SRPKs: Researchers should focus on developing small molecules that can specifically inhibit the activity of SRPKs. These drugs can be used as a single treatment or in combination with other therapies to target cancer cells.
(C) Identifying SRPKs as treatment targets for specific cancer types: Certain SRPKs may be more important in certain types of cancer than others. Future research may focus on determining which SRPKs are most crucial in specific cancer types and developing therapies targeting these enzymes.
(D) Exploring the use of RNA interference (RNAi) to target SRPKs: RNAi is a process that can silence specific gene expression at the RNA level. Researchers should explore using RNAi to target SRPKs as a potential cancer treatment method.
(E) Evaluating the combination of SRPK inhibitors with other therapies: SRPK inhibitors may be most effective when used in combination with other cancer therapies, such as chemotherapy or immunotherapy. Future research may focus on determining the most effective combination therapies, including SRPK inhibitors.
New Potential Target in Cancer Treatment: SRPKs
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



