Advances Challenges and Prospects in Therapeutic Cancer Vaccines
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Advances Challenges and Prospects in Therapeutic Cancer Vaccines: A Comprehensive Overview
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Advances Challenges and Prospects in Therapeutic Cancer Vaccines: A Comprehensive Overview
In recent decades, the landscape of cancer immunotherapy has undergone profound changes with the development and regulatory approval of immune checkpoint inhibitors and adoptive cell therapies.
A recent significant review titled “Therapeutic cancer vaccines: advancements, challenges, and prospects” published in the journal “Signal Transduction and Targeted Therapy,” sheds light on the promising developments in therapeutic cancer vaccines.

Background of the Study:
Malignant tumors remain a leading cause of death, underscoring the urgency to overcome this global challenge. Traditional cancer therapies, including surgery, radiotherapy, and chemotherapy, exhibit significant toxicity and limited applicability, emphasizing the need for more effective treatment methods. Recent research highlights the intricate connection between cancer progression and “cancer immune editing,” suggesting that the immune system can clear newly formed cancer cells by recognizing oncogenic mutations or fostering an immune-suppressive microenvironment conducive to tumor proliferation.
In the era of immunotherapy, various novel approaches, such as immune checkpoint inhibitors (ICIs), oncolytic viruses, and chimeric antigen receptor T-cell (CAR-T) therapies, have gained clinical approval. Despite the success of ICIs, with only 12% of patients benefiting from treatment, exploring alternative immunotherapeutic strategies, including therapeutic cancer vaccines, has become a focus of attention.
Advancements:
Therapeutic cancer vaccines hold the potential to stimulate the generation of cytotoxic T cells effective against tumor cells. Identification of new antigens using advanced technologies like mass spectrometry (MS) and whole exome sequencing (WES)/RNA-seq has paved the way for the development of personalized immunotherapies. Recent studies demonstrate the efficacy of mRNA-based neoantigen vaccines in inhibiting melanoma recurrence, leading to prolonged progression-free survival (PFS). Combining a personalized mRNA cancer vaccine with pembrolizumab significantly reduces the risk of melanoma recurrence or death without a notable increase in severe side effects, earning FDA breakthrough therapy designation. The synergy between neoantigen vaccination and ICI treatment suggests a potential cooperative effect in controlling disease progression.
Challenges:
Patients with minimal residual disease, less affected by the production pipeline timeline, and with incompletely established immune mechanisms inhibiting tumors may be more suitable for vaccine applications. However, challenges persist in designing optimal delivery platforms, selecting the best combination therapies, avoiding immune escape, and inducing robust T-cell immune responses, all of which will be comprehensively addressed in the following review.
Despite observed increases in anti-tumor effector cells following vaccination, clinical benefits have been limited in small cohorts. The review emphasizes current clinical challenges associated with cancer vaccines, considering the complexity of the human immune system and the tumor microenvironment.
Production of cancer vaccines poses a significant challenge. Shared antigen cancer vaccines offer batch production advantages, reducing production time and costs.
However, personalized neoantigen vaccines, considered individual drugs, require rapid, scalable, and cost-effective production methods for swift clinical application.
Synthetic vaccine technologies, featuring simple, robust, invariant, and Good Manufacturing Practice (GMP)-compliant processes, are crucial for addressing these complexities.
Advancements in fully digitized production processes, platform-based quality control, modularized production facilities, and intelligent production hardware offer viable solutions, allowing the creation of large-scale, parallel, and small-scale production lines and significantly improving the overall efficiency and accessibility of cancer vaccine clinical applications.
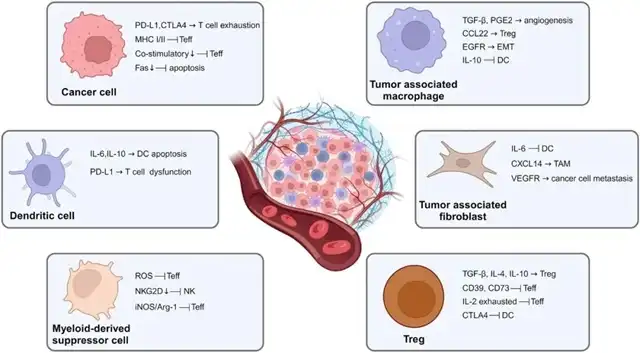
The role of tumor cells and immunosuppressive cells in the tumor immune microenvironment
Prospects:
Future developments in the clinical efficacy of therapeutic cancer vaccines focus on key areas, including the identification of immunogenic neoantigens, optimization of carriers and delivery platforms, and overcoming the immunosuppressive tumor microenvironment (TME). Next-generation sequencing, enhanced computational capabilities, and advanced algorithms make the identification of highly immunogenic neoantigens possible.
Ongoing research aims to improve vaccine technologies, exploring different expression forms, enhancing common stimulatory components, and determining suitable adjuvant methods. The optimal selection of delivery systems is crucial for enhancing antigen immunogenicity and facilitating the entry of antigens and activation signals into antigen-presenting cells (APCs).
Strategic combinations of immune checkpoint inhibitors with other treatment modalities represent another avenue of exploration. These combination approaches may counteract the immune-suppressive environment of the TME and alleviate the development of immunotherapy resistance. However, the precise composition, sequence, and dosage of combination therapies require further research and refinement.
Reference: Nature Article
Advances Challenges and Prospects in Therapeutic Cancer Vaccines: A Comprehensive Overview
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



