CpAMs: New direction of Hepatitis B drug target
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
CpAMs: New direction of Hepatitis B drug target
CpAMs: New direction of Hepatitis B drug target. Hepatitis B is in the direction of new drug target CpAMs, 3 items enter phase I and 5 items enter phase II. A direct-acting antiviral drug development direction is the core protein allosteric modulator.
Hepatitis B is in the direction of new drug target CpAMs, 3 items enter phase I, 5 items enter phase II
Researchers around the world have discovered that the core protein of hepatitis B virus is necessary for HBV-pgRNA packaging and reverse transcription. The target is CpAMs, core protein allosteric modulators. It has also been noted in some medical literature and journals: The target is called capsid assembly modulator. In fact, they all mean the same thing. This compound is being studied for new hepatitis B drugs. According to the chemical structure of CpAMs, unstable abnormal capsids or empty capsids can be formed. At present, according to this mechanism of action, there are 2 categories that have been identified globally.
Respectively, type I CpAMs represented by heteroaryldihydropyridines can lead to the formation of incorrectly assembled capsids by increasing the kinetics of capsid formation. There are also type II CpAMs, mainly represented by phenylpropanamide, which can accelerate the assembly of capsids and form a normal-shaped empty shell, lacking viral pgRNA and HBV polymerase. nvr3-778, it is the world’s first core protein allosteric modulator (CpAMs) evaluated individually and in combination with PegIFN.
For example, in a previous phase 1 clinical trial of chronic hepatitis B patients without liver cirrhosis (e antigen-positive people), the NVR 3-778+pegylated interferon group decreased HBV-DNA and HBV-RNA The amplitude is greater than NVR 3-778 or the peginterferon group alone. However, hepatitis B virus rebound was observed after drug withdrawal. There is another type II CpAM, namely JNJ-6379 (JNJ-56136379). JNJ-6379 mainly binds to the HBV core protein and destroys the early and late stages of the HBV life cycle.
In a previous phase 1 clinical trial for newly treated patients with chronic hepatitis B, administration of JNJ-6379 for 4 weeks proved to be tolerable and showed a dose-dependent pharmacokinetics with strong antiviral activity (HBV-DNA and HBV-RNA are reduced). Here, Xiaofan Health briefly introduces the mechanism of action of the new hepatitis B drug JNJ-6379. JNJ-6379, by interfering with the kinetics of capsid assembly, prevents pgRNA from embedding and blocking HBV replication (the later step of the hepatitis B virus life cycle).
At the same time, JNJ-6379 also inhibits the formation of virus replication template cccDNA, which may interfere with the disintegration of the capsid. However, since JNJ-6379 does not target the hepatitis B surface antigen, its subviral particles can still be released into the blood. JNJ-6379 is currently undergoing clinical phase 2 research. There is also a class I HBV-CpAM, the new drug RO7049389 for hepatitis B that is administered orally, which can induce the formation of abnormal HBV aggregates.
In the latest phase 1 clinical study of RO7049389, the drug was administered to HBV patients with different dosage regimens for 28 days, which can lead to a significant decrease in the median HBV-DNA (2.7 to 3.0 log10 IU/mL), and hopefully inhibit hepatitis B virus. After stopping treatment, the researchers observed a rebound of the virus. Recently introduced the latest progress of global innovative drug-related target trials for hepatitis B, including Bulevirtide (HBV Phase II), NVR 3-778 (Phase I), RO7049389 (Phase I), JNJ-6379 (Phase II), etc.
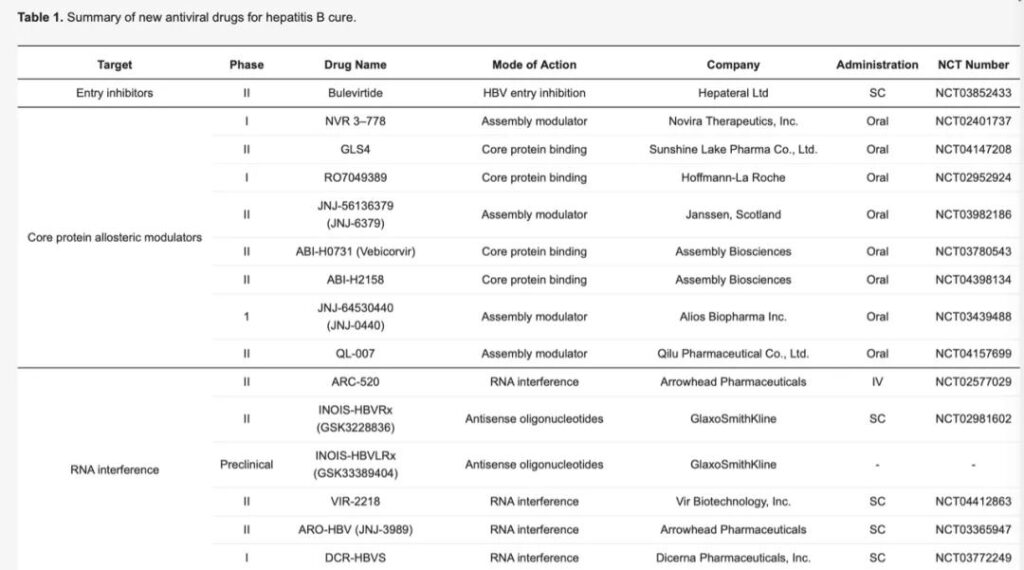
The above picture is from the Department of Internal Medicine, Yonsei University School of Medicine, Seoul, South Korea, the Institute of Gastroenterology, Yonsei University School of Medicine, Seoul, South Korea, and the Yonsei Liver Center of Seoul Severance Hospital, South Korea. They are published in the International Journal of Molecular Sciences It can be seen that there are currently 8 new drug clinical trials based on the target of core protein allosteric modulators (CpAMs), of which 3 have entered the human clinical phase 1, and 5 have entered the human clinical phase 2 (as of December 2020 28th).
(source:chinanet, reference only)
Disclaimer of medicaltrend.org



