Johnson & Johnson announces results of adenovirus COVID-19 vaccine trials
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Johnson & Johnson announces results of adenovirus COVID-19 vaccine trials
Johnson & Johnson announces results of adenovirus COVID-19 vaccine trials. NEJM: Johnson & Johnson’s adenovirus vaccine clinical trial results announced, safe and effective in both young and senior people.

Adenovirus vector vaccine is a vaccine made by recombining protective antigen genes into the adenovirus genome using adenovirus as a carrier, and using recombinant adenovirus that can express protective antigen genes. Adenovirus vector vaccine has sufficient safety and can produce good immunity. Previously, Academician Chen Wei led the research and development of a new coronavirus vaccine based on human adenovirus serotype 5 (Ad5) vector.
On January 13, 2021, the international top medical journal “New England Journal of Medicine” (NEJM) published the interim results of the Phase I/IIa clinical trial of the adenovirus COVID-19 vaccine developed by Johnson & Johnson (Johnson & Johnson).
The vaccine uses a human adenovirus serotype 26 (Ad26) vector, which encodes a full-length and stable new coronavirus S protein.
This interim analysis shows that the Ad26.COV2.S vaccine is safe and immunogenic in both young people (18-55 years old) and elderly people (65 years and older). This result, combined with previous preclinical results, supports the continuation of two phase 3 clinical trials (NCT04505722 and NCT04614948) to evaluate the efficacy of low-dose single-dose injection or two-dose injection regimen (low dose is 5×10E10 Virus particles/ml).
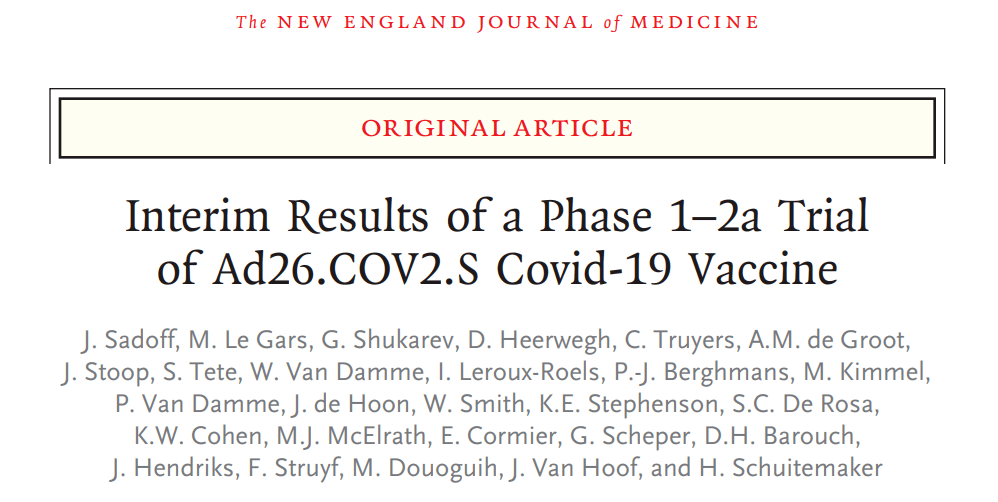
Participants’ situation
In this multicenter, placebo-controlled Phase 1-2a clinical trial, healthy adults aged 18 to 55 (cohort 1) and over 65 years of age (cohort 3) were randomly assigned to receive Ad26.COV2.S vaccine. The dose group was 5×10E10 virus particles/ml, and the high-dose group was 1×10E11 virus particles/ml. One or two doses were injected. Cohort 2 collected long-term data comparing the single-dose schedule and the two-dose schedule. The primary endpoint is the safety and reactogenicity of each dosage regimen.
Cohort 1 (18-55 years old, 402 people) received 2 doses, cohort 3 (65 years and older, 403 people) received 1 dose, after the first dose of vaccine in cohorts 1 and 3, and cohort 1 After the second dose of the vaccine, the most common adverse reactions were fatigue, headache, muscle pain and pain at the injection site. The most common systemic adverse event is fever.
Adverse events in cohort 3 were less common than cohort 1. Adverse events in low-dose vaccine patients were less common than those in high-dose vaccines, and the reactogenicity was lower after the second dose.
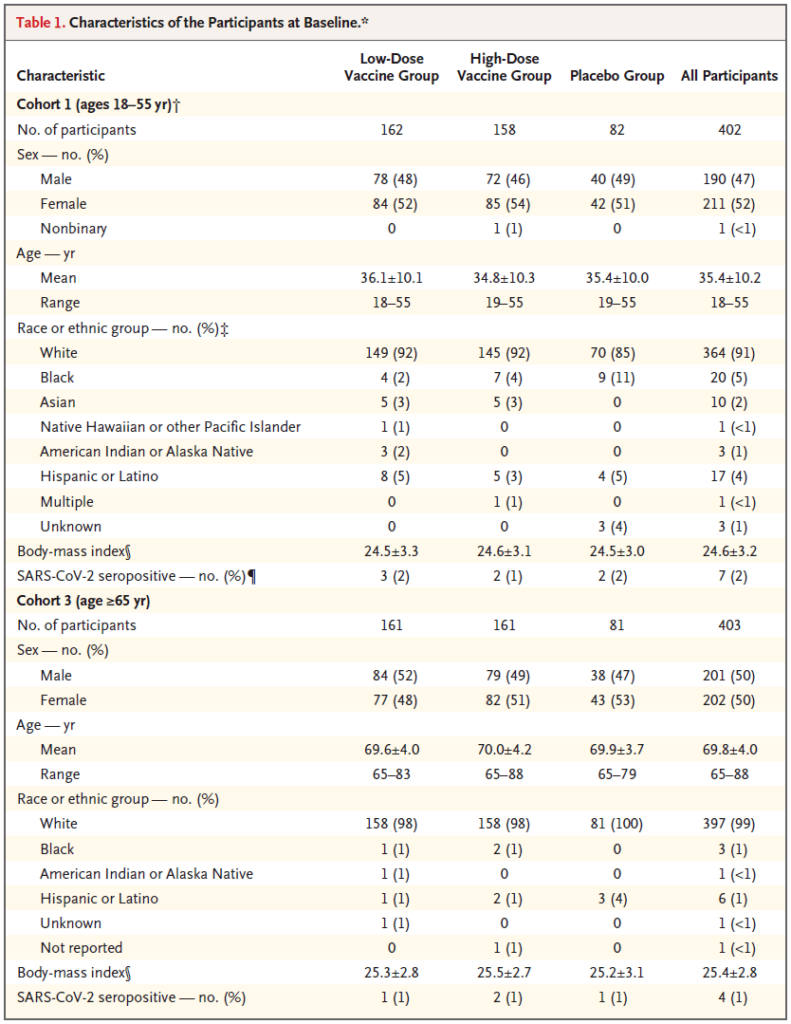
Regardless of the vaccine dose and age group, on the 29th day after the first dose of vaccine, more than 90% of all participants detected neutralizing antibody titers against wild-type virus (geometric mean titers were 224 to 354 ). Neutralizing antibodies were detected in 100% of participants on day 57, and the titer increased further (geometric mean titer was 288 to 488). The neutralizing antibody titer remained stable until at least day 71.
After refracting the second dose of vaccine, the neutralizing antibody titer can be increased to 2.6 to 2.9 times the original (geometric mean titer is 827 to 1266).
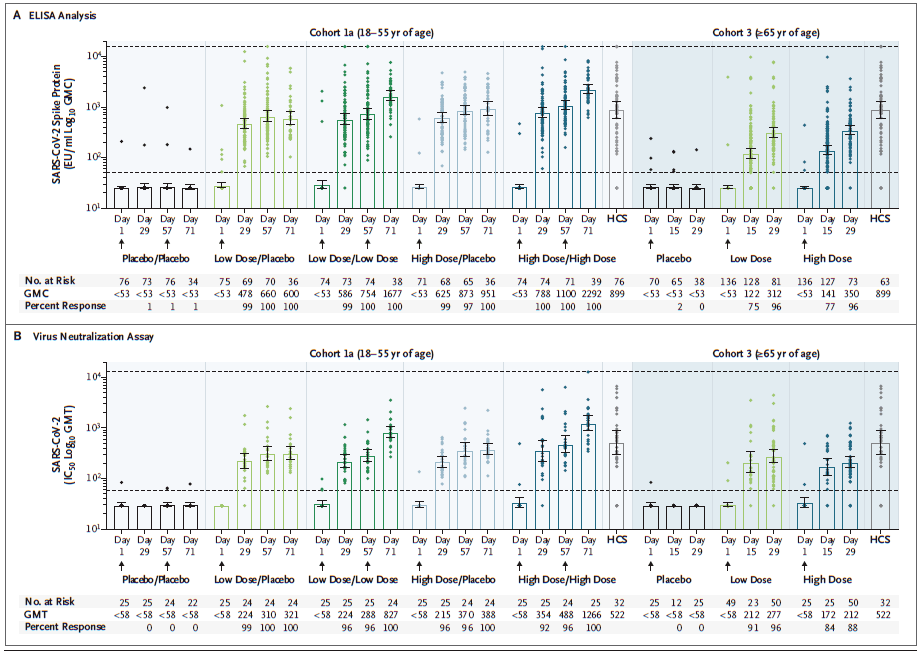
On day 14, 76%-83% of participants in cohort 1 and 60% to 67% of participants in cohort 3 detected CD4+ T cell responses. In general, the CD8+ T cell response was strong, but it was lower in Cohort 3.
This interim analysis shows that the Ad26.COV2.S vaccine is safe and immunogenic in both young people (18-55 years old) and elderly people (65 years and older). This result, combined with previous preclinical results, supports the continuation of two phase 3 clinical trials (NCT04505722 and NCT04614948) to evaluate the efficacy of low-dose single-dose injection or two-dose injection regimen (low dose is 5×10E10 Virus particles/ml).
(source:internet, reference only)
Disclaimer of medicaltrend.org



