2021 ASCO-GI Hepatobiliary Cancer Special Report
- EB Virus Could Be Infected by Kiss: A Hidden Threat Linked to Cancer
- The Silent Threat: How Gas Stoves Pollute Our Homes and Impact Health
- Paternal Microbiome Perturbations Impact Offspring Fitness
- New Report Casts Doubt on Maradona’s Cause of Death and Rocks Manslaughter Case
- Chinese academician unable to provide the exact source of liver transplants
- Early Biomarker for Multiple Sclerosis Development Identified Years in Advance
2021 ASCO-GI Hepatobiliary Cancer Special Report
2021 ASCO-GI Hepatobiliary Cancer Special Report. Affected by the COVID-19 epidemic, the world’s top academic event in the field of gastrointestinal oncology, the 2021 American Society of Clinical Oncology Gastrointestinal Oncology Symposium (ASCO-GI) virtual meeting will be held on January 15-17, 2021 local time 23:00 on the 16th) Grand opening.
Affected by the COVID-19 epidemic, the world’s top academic event in the field of gastrointestinal oncology, the 2021 American Society of Clinical Oncology Gastrointestinal Oncology Symposium (ASCO-GI) virtual meeting will be held on January 15-17, 2021 local time 23:00 on the 16th) Grandly held, the theme of the conference is “Discover More”. This article selected 10 abstracts related to liver and gallbladder cancer from the conference and translated them into four parts: dual immunization combination, immune combination targeting, targeting combined topical and single-drug targeted therapy, in order to provide experts in the field of liver and gallbladder cancer. Clinical diagnosis and treatment and scientific research work have been helpful.
2021 ASCO-GI Hepatobiliary Cancer Special Report.
2021 ASCO-GI Hepatobiliary Cancer Special Report.
Double Immunity Combination—-2021 ASCO-GI Hepatobiliary Cancer Special Report
Abstract 313: Exposure-response (E-R) efficacy and safety (E-S) analyses of tremelimumab as monotherapy or in combination with durvalumab in patients (pts) with unresectable hepatocellular carcinoma (uHCC)
Analysis of the exposure response (E-R) efficacy and safety (E-S) of Tremilimumab alone or in combination with duvalvumab in patients with unresectable hepatocellular carcinoma (uHCC)
Author: Xuyang Song, AstraZeneca, Gaithersburg, MD
background
In the phase II study of uHCC (Study 22, NCT02519348), a combination of the new Tremelimumab (T; anti-CTLA-4 monoclonal antibody) and duvalizumab (D; anti-PD-L1 monoclonal antibody) (T300 + D) Comparison of the two single-drug regimens or the T75+D combination regimen shows good clinical efficacy. This article analyzes and evaluates the pharmacokinetics (PK) of T monotherapy or combination therapy in the study and the relationship between drug exposure and safety, efficacy, and pharmacodynamics (PD).
method
A total of 216 patients were included in the analysis (T, 65 cases; T300 + D, 72 cases; T75 + D, 79 cases). Standard pharmacological methods were used to analyze safety, anti-tumor activity, PK, PD and immunogenicity. Using the previously established independent PK model for T monotherapy for solid tumors, the T monotherapy and combination therapy data in Study 22 were used for analysis, including post-covariate analysis to assess the impact of covariates on various PK parameters. The population PK and PD models involve single-agent T exposure to safety parameters, PD and effectiveness (overall survival, OS; progression-free survival, PFS; objective response rate, ORR). The Kaplan-Meier method was used to explore the relationship between E-R and PFS and OS, and the Cox proportional hazard model (CPHM) was used to analyze.
result
For the single-agent T and T+D combination, for grade 3/4 treatment-related adverse events (TRAE), grade 3/4 TRAE of special concern and AEs that led to treatment interruption, no significant correlation with E-S was observed. The results of the analysis of the T exposure for each quartile showed that patients with higher exposures (third and fourth quartiles) may have longer OS. CPHM analysis showed that if prognostic factors (baseline albumin and neutrophil to lymphocyte ratio) are taken into account, AUC and Cmin are not factors that significantly affect OS. In patients treated with T monotherapy, there was no significant correlation between response and ORR and PFS and any PK exposure indicators of monotherapy. The saturable relationship (described by Emax) was observed to have the greatest change in proliferative T cell counts in the exposed group compared to baseline.
in conclusion
The observed PK data is basically consistent with the prediction of the PK model in the historical population, suggesting that the PK of single-agent T in uHCC patients is consistent with that in other solid tumors. No significant relationship was found between E-S and E-R; therefore, PK is not an important predictor of the efficacy or safety of single-agent T. Even considering the limitation of sample size, the saturation relationship is still observed in the proliferating T cells to support a single dose of T300. In the future, data collected from Study 22 and the larger Phase III HIMALAYA study (NCT03298451) will be analyzed to further explore E-R and develop a T300 + D combination program.
※AstraZeneca’s Tremilimumab single-drug or combined Durvalumab has not been approved in China, and the content of this information is not intended as a treatment or use recommendation. The current indication for Durvalumab approved in China is after receiving platinum-based chemotherapy and concurrent radiotherapy Treatment of unresectable, stage III non-small cell lung cancer (NSCLC) patients without disease progression.
Abstract 269: Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): Long-term results from CheckMate 040.
Nivolumab (NIVO) combined with ipilimumab (IPI) for patients with advanced hepatocellular carcinoma (aHCC): long-term results of CheckMate 040
Author: Anthony B. El-Khoueiry, USC Norris Comprehensive Cancer Center, Los Angeles, CA
background
According to the preliminary results of CheckMate 040 (NCT01658878) study: the objective response rate (ORR) is 32%,
The median overall survival (mOS) is 22.8 months (mo)1, NIVO 1 mg/kg + IPI 3 mg/kg Q3W (4 doses), followed by NIVO 240 mg Q2W is approved in the United States for past claims Rafenib-treated aHCC patients. We provide here the 44-month long-term follow-up results from the CheckMate 040 NIVO+IPI cohort.
method
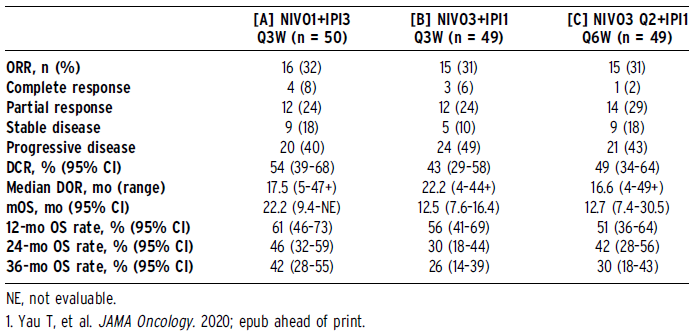
The patients were randomly divided into 3 groups: [A] NIVO 1 mg / kg + IPI 3 mg / kg Q3W (4 doses) or [B] NIVO 3 mg / kg + IPI 1 mg / kg Q3W (4 doses), each group Followed by NIVO 240 mg Q2W, or [C] NIVO 3 mg / kg Q2W + IPI 1 mg / kg Q6W. Continue treatment until intolerable toxicity or disease progression. Evaluate safety, tolerability, ORR (a blinded independent center review based on RECIST v1.1), duration of remission (DOR), disease control rate (DCR) and OS. The data deadline is May 26, 2020.
result
148 patients were randomized. The minimum follow-up time is 44 months. The mOS of group A was 22.2 months, group B was 12.5 months, and group C was 12.7 months; the 36-month OS rates were 42%, 26%, and 30%, respectively. Each treatment group achieved a durable response, and some cases had DOR close to 4 years. The DCR of group A was higher than that of group B and C. Regardless of the baseline programmed death ligand 1 expression (<1% or ≥1%), or the baseline alpha-fetoprotein level (<400 mg/L or ≥400 mg/L), a response can be observed. In groups B and C, patients with hepatitis B or C virus (HBV or HCV) had higher ORR than uninfected patients (29% vs 43% vs 9% in group B, 31% vs 42% vs 0% in group C ). ORR in group A has nothing to do with the cause (HBV, 32%; hepatitis C virus, 29%; uninfected, 31%). See the table for additional efficacy data. Since the initial analysis, there have been no additional discontinuations due to treatment-related adverse events or immunopharmacological adverse events (IMAEs). According to reports, group A has more IMAEs than groups B and C; the most common are skin rash, hepatitis and adrenal insufficiency. Most IMAEs are reversible and can be reversed and resolved after treatment with established methods.
in conclusion
During at least 44 months of follow-up, the second-line NIVO1+IPI3 continued to show clinically significant responses and long-term survival benefits in aHCC. Safety is controllable, and no new safety signals were found in the long follow-up.
Abstract330: Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors.
Ipilimumab combined with nivolumab/pembrolizumab for advanced liver cancer refractory to immune checkpoint inhibitors
Author: Jeffrey Sum Lung Wong, University of Hong Kong, Hong Kong, China
background
The use of immune checkpoint inhibitors (ICI) to block the programmed cell death protein 1 (PD-1) pathway is currently the standard treatment for advanced hepatocellular carcinoma (HCC). There is currently no strategy to overcome ICI resistance. Our purpose is to evaluate the efficacy of ipilimumab combined with anti-pd-1 ICIs (Nivolumab or Pembrolizumab) in advanced HCC patients with advanced ICIs.
method
Patients with advanced liver cancer developed tumor progression after previous ICI treatment and subsequently received ipilimumab combined with nivolumab/pembrolizumab. To evaluate the objective response rate (ORR), median time to response (DOR), time to progression (TTP), overall survival (OS), and treatment-related adverse events (TRAEs).
Result
A total of 25 patients were enrolled, with a median age of 62 years (51-83 years). 68% of patients were Child-Pugh (CP) grade A, and 48% of patients were resistant to the previous ICI. At a median follow-up of 37.7 months, the ORR was 16% and the median DOR was 11.5 months (range 2.76-30.3). Three patients achieved complete remission. The median TTP was 2.96 months (95% C.I. 1.61-4.31). The median OS was 10.9 months (95% C.I. 3.99-17.8), and the 1-, 2-, and 3-year survival rates were 42.4%, 32.3%, and 21.6%, respectively. The ORR of the original resistance group was 16.7%, and the ORR of the acquired resistance group was 15.4% (p=1.00). All responders were CP A and albumin-bilirubin (ALBI) grade 1 or 2. CP and ALBI grades were significantly correlated with OS (p=0.006 and P<0.001). Overall, 52% of patients had TRAE, and 12% had grade 3 or higher TRAE.
Conclusion
Ipilimumab combined with nivolumab/pembrolizumab can achieve long-lasting anti-tumor activity, improve the survival outcome of advanced HCC patients who have previously received ICIs, and have tolerable toxicity. This result is encouraging.
Immune Combination Targeting—-2021 ASCO-GI Hepatobiliary Cancer Special Report
Abstract 326: Safety and efficacy of combination of GT90001, an anti-activin receptorlike kinase-1 (ALK-1) antibody, and nivolumab in patients with metastatic hepatocellular carcinoma (HCC).
The effectiveness and safety of ALK-1 inhibitor GT90001 combined with nivolumab in the second-line treatment of metastatic hepatocellular carcinoma
Author: Chiun Hsu, Department of Medical Oncology, National Taiwan University Cancer Center, Taipei, Taiwan
background
GT90001, an anti-ALK-1 monoclonal antibody (IgG2) that can inhibit the ALK-1/TGF-b signaling pathway and tumor angiogenesis, has shown good single-agent safety in humans. This study aims to evaluate the safety and efficacy of GT90001 combined with nivolumab in the treatment of a clue of rafenib or lenvatinib in patients with advanced HCC who have advanced or intolerant disease (NCT03893695).
method
We conducted a phase I/II, open-label, one-arm, dose-amplification study in three centers in Taiwan to explore the use of GT90001 combined with nivolumab for HCC patients diagnosed with histology or cytology. The liver function of the patient is Child. -PughA grade, ECOG score of 0-1 points, patients who have previously received immune checkpoint inhibitor therapy cannot be included in the group. The first phase (dose expansion phase) evaluated three different doses of GT90001 (7 (starting dose), 4.5, and 3 mg/kg, Q2W) in 6 patients in combination with nivolumab 3 mg/kg Q2W . Dose-limiting toxicity (DLT) is defined as any treatment-related grade 3-4 adverse event that occurs within 28 days of the first treatment. The second phase (expansion cohort) included 14 patients who received treatment until they lost clinical benefit or intolerable toxicity. The primary study endpoint is the ORR assessed by the investigator according to RECIST v1.1.
result
From July 9, 2019 to June 26, 2020, 20 eligible patients were included. No DLT was observed in the first stage, and GT90001 7.0 mg/kg + pembrolizumab 3.0 mg/kg was given every 2 weeks in the second stage. Common AEs (incidence greater than 20%) include decreased platelet count, skin rash, fatigue, dizziness, peripheral edema, and constipation. Three patients had treatment-related serious AEs (renal insufficiency, autoimmune hepatitis, hyperamylaseemia). The median follow-up time was 3.7 months. Among the 16 evaluable patients, objective responses were observed in 7 patients, all of which were partial remissions, and 4 of them were confirmed. The disease control rate was 56.2%. The updated efficacy data will be displayed at the conference.
in conclusion
The combined application of GT90001 and Nivolumab showed manageable safety, and no new safety signals were found. The preliminary anti-tumor activity shows the potential of GT90001 combined with nivolumab as a second-line treatment for advanced HCC.
Abstract 267: IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC ).
IMbrave150:: Updated overall survival in a global, randomized, open-label phase III study of atzolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) for patients with unresectable hepatocellular carcinoma (HCC) Period (OS) data.
Author: Richard S. Finn, Jonsson Comprehensive Cancer Center, Geffen School of Medicine at UCLA, Los Angeles, CA
background
According to the results of IMbrave150 (NCT03434379), Atezo + bev has been approved globally for unresectable HCC patients who have not previously received systemic therapy. During a median follow-up of 8.6 months, two primary endpoints were reached, and atezo + bev vs sor was observed in OS (HR, 0.58 [95% CI, 0.42, 0.79]; P, 0.001) and independently assessed progression-free survival Period (PFS; 1.1 per RECIST; HR, 0.59 [95% CI, 0.47, 0.76]; P, 0.001) has a statistically significant and clinically significant improvement (Fern et al., British Medical Journal 2020). Here, we report the updated IMbrave150 OS analysis.
method
In this global, multicenter, randomized, open-label phase III study IMbrave150, 501 unresectable HCC patients who received systemic treatment for the first time (≥1 measurable untreated disease RECIST 1.1, Child-Pugh A Liver function and ECOG PS 0/1). Randomly receive atezo 1200 mg IV q3w + bev 15 mg/kg IV q3w or sor 400 mg bid according to 2:1 until unacceptable toxicity or loss of clinical benefit assessed by researchers. This postmortem descriptive OS analysis was performed during an additional follow-up 12 months after the initial analysis.
result
501 patients were included, including 336 atezo + bev and 165 sor. As of August 31, 2020, the median follow-up was 15.6 months, and 280 OS events were observed. The median overall survival in the atezo + bev group was 19.2 months and that in the sor group was 13.4 months (HR, 0.66 [95% CI, 0.52, 0.85]; P = 0.0009). The 18-month survival rate was 52% in the atezo + bev group and 40% in the sor group. The survival benefit of atezo + bev compared to sor was basically the same in the subgroup and preliminary analysis. The updated objective response rate was consistent with the initial analysis (29.8% in the atezo + bev group), and more patients achieved complete remission (CR; 7.7%). Other response data are in the table. The safety is consistent with the initial analysis and no new signals have been found. .
in conclusion
During the 12-month follow-up, IMbrave150 showed consistent clinical treatment benefits and safety. In the first-line phase III study of advanced liver cancer, the combination of atezo + bev obtained the longest survival period, which confirmed that atezo + bev can be used as the standard treatment for untreated, unresectable liver cancer.
Abstract 321: Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study.
Multi-cohort phase II study of lenvatinib combined with pembrolizumab in the treatment of treated patients with biliary system tumors: LEAP-005
Author: Luis Villanueva, Fundaci’on Arturo L’opez P’erez, Providencia, Santiago, Chile
background
The second-line treatment options for patients with biliary system tumors (BTC) are limited. Lenvatinib is an anti-angiogenic multi-target tyrosine kinase inhibitor, combined with immune checkpoint inhibitor pembrolizumab for advanced entities Tumor has shown effective anti-tumor activity. The LEAP-005 (NCT03797326) study evaluated lenvatinib combined with pembrolizumab in the treatment of patients with advanced treated solid tumors. Here we report the results of the BTC cohort of the LEAP-005 study.
method
In this non-random, open-label phase II study, eligible patients were under 18 years of age, histologically or cytologically confirmed (metastatic and/or unresectable) BTC, who had disease progression after first-line treatment and had Measurable lesions assessed according to RECIST v1.1. The ECOG score is 0-1, which can provide tumor tissue samples for detecting PD-L1 expression.
The enrolled patients received lenvatinib 20mg QD combined with pembrolizumab 200mg Q3W, and the longest treatment period was 35 cycles (about 2 years), or reached imaging confirmed disease progression or intolerable toxicity or The patient withdrew consent. For patients with clinical benefit, lenvatinib treatment can last for more than 2 years.
The primary endpoints are ORR (according to RECIST v1.1, assessed by an independent blind center) and safety. Secondary endpoints are disease control rate (DCR; including CR, PR and SD), duration of response (DOR), PFS and OS. Tumor evaluations were performed every 9 weeks from the start of treatment for 54 weeks, then every 12 weeks until the 104th week, and every 24 weeks thereafter.
result
A total of 31 patients were enrolled in the BTC cohort (ECOG score of 1.55%; extrahepatic metastasis-unresectable, 84%). As of April 10, 2020, the median time from the first dose to the data cutoff (DCO) was 9.5 months (range: 3.1±11.9), 16 patients received treatment, and 3 (10%) patients PR was achieved and 18 (58%) patients achieved SD. ORR was 10% (95% CI, 2‒26), and DCR was 68% (95% CI, 49‒83). The median DOR was 5.3 months (range from 2.1+ to 6.2). The median PFS was 6.1 months (95% CI, 2.1‒6.4). The median OS was 8.6 months (95% CI, NR-5.6). Treatment-related adverse events occurred in 30 patients (97%), including grade 3 to 4 adverse events in 15 patients (48%). No treatment-related deaths were observed. Two patients (6%) discontinued treatment due to treatment-related AEs (1 case of myocarditis, 1 case of fever). The most common AEs related to treatment were hypertension (42%), speech impairment (39%), diarrhea (32%), fatigue (32%) and nausea (32%). Fourteen patients (45%) had immune-related AEs, and 1 patient (3%) had an infusion reaction.
in conclusion
Lenvatinib combined with pembrolizumab has shown encouraging efficacy and manageable toxicity in advanced BTC patients who have previously received first-line treatment progress. Based on these data, the enrollment of the BTC cohort has been expanded to 100 patients.
Single-drug Immunization/Targeting—2021 ASCO-GI Hepatobiliary Cancer Special Report
Abstract 315: Safety profile of immune checkpoint inhibitors versus sorafenib as firstline treatment in advanced hepatocellular carcinoma: A meta-analysis of randomized controlled trials
Safety of immune checkpoint inhibitors and sorafenib as first-line treatment of advanced hepatocellular carcinoma: a meta-analysis based on randomized controlled studies
Author: Alessandro Rizzo, Department of Experimental, Diagnostic, and Specialty Medicine-DIMES, Sant’Orsola- Malpighi Hospital, University of Bologna, Bologna, Italy
background
Systemic therapy represented by tyrosine kinase inhibitors (such as sorafenib) is the main treatment for advanced hepatocellular carcinoma (HCC). However, the overall survival benefit is still disappointing, mainly due to the generation of acquired drug resistance and poor safety, so it is often necessary to adjust the treatment plan or terminate the treatment early, which affects the patient’s compliance and long-term Prognosis. Immune checkpoint inhibitors (ICIs) are rapidly developing as a new treatment option for advanced HCC, and the toxicity characteristics of these drugs should be kept in mind. This meta-analysis aims to compare the adverse events (ADEs) of ICIs (monotherapy or in combination with other anticancer drugs) in the first-line treatment of advanced hepatocellular carcinoma versus sorafenib monotherapy.
method
Eligible randomized controlled studies include ICIs versus sorafenib as the first-line treatment for HCC. Statistics and analysis of all levels of ADEs in all included research reports. The results are as follows: itching, diarrhea, hand-foot-skin reaction (HFSR), fatigue, increased aspartate aminotransferase (AST), skin rash, high blood pressure and decreased appetite. By calculating the odds ratio (OR) with 95% confidence interval (CI); the calculation of OR is combined with the Mantel-Haenszel method. All statistical analyses were performed using R-studio software.
result
A total of two randomized controlled studies (CheckMate 459, IMbrave 150) were analyzed, with a total of 1,228 patients. The results suggest that patients treated with ICIs have a higher risk of itching (OR 1.99, 95% CI = 1.22-3.24), while sorafenib treatment has a higher risk of diarrhea (OR 0.26, 95% CI = 0.18-0.37) and HFSR (OR 0.01, 95% CI = 0-0.04) risk. Conversely, in fatigue (OR 0.84, 95% CI = 0.45-1.58), AST increased (OR 1.21, 95% CI = 0.78-1.88), skin rash (OR 0.71, 95% CI = 0.46-1.11)), hypertension (OR 0.28, 95% CI = 0.01-9.76) and decreased appetite (OR 0.41, 95% CI = 0.14-1.21), no statistically significant difference was observed between the two groups.
in conclusion
Although patient heterogeneity will affect our analysis, the treatment of advanced liver cancer with ICIs is feasible and the toxicity is tolerable. For advanced hepatocellular carcinoma, when choosing a suitable first-line treatment, in addition to considering the effectiveness of the treatment, the toxicity of the treatment should also be carefully considered.
Abstract 297: Pembrolizumab(pembro) monotherapy for previously untreated advanced hepatocellular carcinoma(HCC): Phase II KEYNOTE-224 study.
Pembrolizumab (pembro) monotherapy for previously untreated advanced hepatocellular carcinoma (HCC): Phase II KEYNOTE-224 study
Author: Jean-Luc Van Laethem, Erasme Hospital, Brussels, Belgium
background
The KEYNOTE-224 study, cohort 1 is an open-label, single-arm, international multi-center phase II clinical study. The results show that pembrolizumab is used as a single agent for patients with advanced HCC who have previously received sorafenib treatment It is tolerable and effective. Here, the results of KEYNOTE-224 study cohort 2 are reported, which enrolled patients with advanced HCC who had not received systemic treatment.
method
Cohort 2 included HCC confirmed by histology, cytology or imaging, unresectable HCC that was not suitable or refused to receive local treatment. Liver function is Child-Pugh A, Blind Independent Center Evaluation (BICR) is a measurable lesion defined by RECIST v1.1, ECOG 0-1 points, BCLC C or B stage. The patient received pembrolizumab 200 mg IV Q3W for about 2 years, or until disease progression or intolerable toxicity, or the patient withdrew their informed consent or the investigator’s decision. The primary study endpoint is the ORR assessed by BICR according to RECIST v1.1, and the secondary study endpoints include DOR, DCR, TTP, PFS, OS and safety/tolerability. Tumor assessments are performed every 9 weeks. Evaluate the efficacy and safety of the treatment in patients who received at least one dose of the study treatment. The DoR was evaluated based on the tumor response, and the 95% CI ORR and DCR were calculated using the Colpper-Pearson method. The Kaplan-Meier method is used to calculate OS, PFS and DOR. A sample of about 50 patients can provide acceptable accuracy for ORR evaluation.
result
A total of 51 patients were enrolled in cohort 2 of the study, and the median time from the first dose to the data cutoff (July 31, 2020) was 21 months (range: 17 months to 23 months). The median age of the patients was 68 years (range: 41-91 years). One patient had HBV infection, 80% of patients consumed alcohol, 8% had HCV infection, and 18% had vascular invasion. 35% had extrahepatic metastases, 33% had BCLC stage B, and 67% had BCLC stage C liver cancer. ORR was 16% (95% CI, 7-29), and the results were similar in different subgroups. The median DoR is not reached (range: 3-20 months or more); approximately 70% of patients have a response duration of more than 12 months. No patient achieved complete remission, 8 (16%) patients achieved partial remission, 21 (41%) patients achieved stable disease and 17 (33%) disease progressed. 5 (10%) cases could not be assessed or not assessed. DCR was 57%, median TTP was 4 months (95% CI, 3-8), median PFS was 4 months (95% CI, 2-6), and median OS was 17 months (95% CI , 8-NA). The PFS rate at 18 months was 16%, and the OS rate at 18 months was 46%. Twenty-seven (53%) patients experienced treatment-related adverse events (TRAE); the most common TRAEs were diarrhea, fatigue, hypothyroidism, and myalgia. Grade 3 TRAE occurred in 7 (14%) patients. 6% of patients discontinued treatment because of TRAE, and 11 (22%) patients had immune-related adverse reactions and infusion reactions. One death event related to treatment was myocarditis with immune-related hepatitis.
in conclusion
In patients with advanced HCC who have not received systemic treatment, pembrolizumab monotherapy can provide long-lasting anti-tumor activity, promising overall survival, and has been shown to be compatible with pembrolizumab in advanced HCC. Similar to the safety observed in. These results support the further exploration of first-line treatment options based on pembrolizumab in HCC.
Abstract 268: Pembrolizumab (pembro) vs placebo (pbo) in patients (pts) with advanced hepatocellular carcinoma (aHCC) previously treated with sorafenib: Updated data from the randomized, phase III KEYNOTE-240 study.
Pembrolizumab (pembro) vs placebo (pbo) in patients with advanced hepatocellular carcinoma (aHCC) previously treated with sorafenib: the latest data from the randomized, phase III KEYNOTE-240 study.
Author: Philippe Merle, Hopital de la Croix-Rousse, Hospices Civils de Lyon, Lyon, France
background
KEYNOTE-240 (NCT02702401) confirmed that compared with placebo, the anti-pd-1 antibody pembro has improved OS and PFS in aHCC patients who have previously received sorafenib. However, the study did not meet the predetermined statistical significance criteria for OS and PFS. The median OS (final analysis) was 13.9 months in the pembro group and 10.6 months in the pbo group (HR 0.781; 95% CI 0.611-0.998). In the first interim analysis, when the PFS and ORR were pre-specified for testing, the median PFS of pembro was 3.0 months, while the median PFS of pbo was 2.8 months (HR 0.775; 95% CI 0.609-0.987). The ORR of pembro is 16.9% (CR, n = 3) and the pbo is 2.2% (CR, n = 0). AEs comply with the known safety standards of pembro. After about 1.5 years of follow-up, we reported the long-term data of KEYNOTE-240.
method
Adult patients confirmed to have aHCC and failed (progressive or intolerant) Sorafenib treatment will be randomized 2:1 to receive Pembro 200 mg IV Q3W + best supportive care (BSC) or pbo + BSC, accept ≤ 35 cycles of treatment or until progress/unacceptable toxicity is confirmed, consent is withdrawn, or the investigator decides. The primary dual end points were OS and PFS, which were assessed by the blinded independent central review (BICR) according to RECIST v1.1. Secondary endpoints include ORR, DOR, DCR, TTP (all assessed by BICR according to RECIST v1.1) and safety.
result
Of the 413 patients, 278 were randomized to receive pembro treatment, and 135 received pbo treatment. As of July 13, 2020, the median time from randomization to data cutoff for pembro was 39.6 months (range 31.7-48.8), and pbo was 39.8 months (31.7-47.8). The median OS in the pembro group was 13.9 months (95% CI 11.6-16.0), and the median OS in the pbo group was 10.6 months (8.3-13.5) (HR 0.771; 95% CI 0.617-0.964). The OS rates of pembro and pbo at 24 and 36 months were approximately 28.8% and 20.4%, 17.7% and 11.7%, respectively. The median PFS in the pembro group was 3.3 months (95% CI 2.8-4.1), and the median PFS in the pbo group was 2.8 months (1.6-3.0) (HR 0.703; 95% CI 0.559-0.885). The PFS of pembro and pbo at 24 months is estimated to be 11.8% and 4.8%. The ORR of pembro was 18.3% (95% CI 14.0-23.4), and the pbo was 4.4% (1.6-9.4). The median response time for pembro was 2.7 months (95% CI 1.2-16.9), and the median response time for pbo was 2.9 months (1.1-6.9). The median DOR of pembro was 13.9 months (1.5+-41.9+) and that of pbo was 15.2 months (2.8-21.9); 45.1% of responders in the pembro group and 33.3% of the responders in the pbo group had a DOR ≥12 months. The DCR of the pembro group was 61.9% and that of the pbo group was 53.3%. The best overall curative effect was: 10 CR, 41 PR, 121 SD and 85 PD in the pembro group, 0 CR, 6 PR, 66 SD and 54 PD in the pbo group. The median TTP of pembro was 4.0 months (95% CI 2.8-5.3), and the median TTP of pbo was 2.8 months (1.6-3.0). No new or unexpected AEs occurred. The frequency of immune-mediated hepatitis events assessed by the sponsor did not increase during follow-up. There were no subsequent hepatitis B or C virus outbreaks.
in conclusion
In the previously treated aHCC patients, compared with pbo, pembro maintained the improvement of OS and PFS, and the safety remained consistent. These data support the benefits of pembro.
Targeting combined local articles—-2021 ASCO-GI Hepatobiliary Cancer Special Report
Abstract 270: TACTICS: Final overall survival (OS) data from a randomized, open label, multicenter, phase II trial of transcatheter arterial chemoembolization (TACE) therapy in combination with sorafenib as compared with TACE alone in patients (pts) with hepatocellular carcinoma ( HCC).
A randomized, open-label, multicenter phase II clinical trial showed the final overall survival (OS) data of patients with hepatocellular carcinoma (HCC) treated with transcatheter arterial chemoembolization (TACE) combined with sorafenib and TACE alone
Author: Masatoshi Kudo, Kindai University Faculty of Medicine, Osaka, Japan
background
So far, many trials have been conducted to compare the efficacy and toxicity of TACE combined with molecularly targeted drugs and TACE alone; these studies have shown no clinical benefit in terms of progression-free survival (PFS) or OS. In the TACTICS (NCT01217034) study, TACE combined with sorafenib significantly improved PFS in patients with unresectable HCC than TACE alone. (Gut 2020;69:1374-1376). Here, we report the final OS analysis of the TACTICS clinical trial reaching a predefined mature OS event.
method
Patients with unresectable liver cancer were randomly assigned to TACE combined with Sorafenib (n = 80) or TACE alone (n = 76). Patients in the combination group took sorafenib (400 mg QD) within 2-3 weeks before TACE, and then 800 mg BID during routine TACE until unmanageable progression (TTUP) occurs, which is defined as untreated tumor progression, transient Deterioration to Child-Pugh C or vascular infiltration/intrahepatic spread. The primary endpoint is progression-free survival (PFS), namely TTUP, or the time to death from any cause and OS. Adjust the diversity through gate-level testing.
result
As of July 31, 2020, 131 OS events have been observed. The median OS in the TACE combined with sorafenib group was 36.2 months, compared with 30.8 months in the TACE group alone (HR, 0.861 [95% CI, 0.607, 1.223]; P = 0.40). ΔOS is 5.4 months. The updated PFS of the TACE combined with sorafenib treatment group was 22.8 months, while the TACE treatment alone was 13.5 months (HR, 0.661 [95% CI, 0.466, 0.938]; P = 0.02). In the TACE combined with sorafenib, 47 cases (58.8%) were observed to be actively treated with topical/drugs after the study, and 58 (76.3%) were observed in the TACE group alone. The anticancer treatment in the TACE group alone included 14 cases of resection/ablation, 53 cases of arterial therapy, and 7 cases of radiotherapy. The use of anticancer drugs in TACE alone included 40 cases (29 sorafenib, 5 regorafenib, 3 lenvatinib, 3 ramolumab), 5 cases of systemic chemotherapy, and 5 cases of immunotherapy. The safety is consistent with the main analysis, and no new signals have been found.
in conclusion
In the TACTICS study, although the PFS of the TACE combined with sorafenib group continued to improve significantly, there was no OS benefit compared with TACE alone. Compared with the first 5 TACE combined trials, the TACTICS trial TACE combined with sorafenib had the longest OS (36.2 months) and the longest ΔOS (5.4 months). It is speculated that the main reason for the negative OS result is the active treatment (76.3%) after many trials in the control group. This means that in the current era when there are many active local and systemic sequential treatments, the OS endpoint of the TACE combined trial may not be Feasible again.
2021 ASCO-GI Hepatobiliary Cancer Special Report
2021 ASCO-GI Hepatobiliary Cancer Special Report
2021 ASCO-GI Hepatobiliary Cancer Special Report.
2021 ASCO-GI Hepatobiliary Cancer Special Report
(source:chinanet. reference only)
Disclaimer of medicaltrend.org